There is an evolving understanding that autoimmune pancreatitis (AIP) is an immunoglobulin (Ig) G4 systemic disease. It can manifest as primarily a pancreatic disorder or in association with other disorders of presumed autoimmune cause. Classic clinical characteristics include obstructive jaundice, abdominal pain, and acute pancreatitis. Thus, AIP can be difficult to distinguish from pancreatic malignancy. However, AIP may respond to therapy with corticosteroids, and has a strong association with other immune mediated diseases. Although primarily a pathologic diagnosis, attempts have been made to reliably diagnose AIP clinically. AIP can be classified as either type 1 or type 2.
Key points
- •
Autoimmune pancreatitis (AIP) can affect the pancreas primarily; however, it can also present as part of a systemic disease related to immunoglobulin G4.
- •
AIP is primarily a histologic diagnosis, but AIP is currently diagnosed using clinical characteristics.
- •
The mainstay of therapy for AIP is corticosteroids. Other therapies that have been explored include immunomodulator drugs.
- •
Relapse rates following corticosteroid therapy are high.
Introduction
Cases of autoimmune pancreatitis (AIP) were described as early as the 1960s by Sarles and colleagues. However, the term autoimmune pancreatitis was first introduced by Yoshida and colleagues in 1995 after these investigators studied a Japanese cohort with causes suggestive of autoimmune origin. Although AIP is recognized as a distinct disease process, the incidence remains unknown. A 2009 survey in Japan estimated that the incidence of AIP in the Ishikawa district (population 1.16 million) was approximately 1 per 100,000. The rate of undiagnosed AIP in large cohorts of patients undergoing pancreatic surgical resection of presumed pancreatic cancer is approximately 2%.
AIP was initially recognized as a disease associated with characteristic clinical, radiologic, and serologic features affecting primarily the pancreas, with the ability to involve other organs. However, more recently AIP has been associated with other immune-mediated diseases, including immunoglobulin (Ig) G4–associated cholangitis (IAC), salivary gland disorders, mediastinal fibrosis, retroperitoneal fibrosis, tubulointerstitial disease and inflammatory bowel disease, and increased levels of IgG4, both in tissue plasma cells and in the serum, thus terming this collection of disease processes IgG4-related systemic disease.
With improved understanding of AIP and its distinct clinical profiles and variable association with a systemic IgG4 disease process, AIP has been classified into type 1 and type 2 AIP. In type 1 AIP, the pancreas is affected as part of a systemic IgG4-positive disease, also known as lymphoplasmacytic sclerosing pancreatitis (LPSP). Type 2 AIP is characterized by histologically confirmed idiopathic duct-centric pancreatitis, often with granulocytic epithelial lesions (GELs) with or without granulocytic acinar inflammation along with absent (0–10 cells per high-power field [HPF]) IgG4-positive cells, and without systemic involvement. Classic clinical characteristics include obstructive jaundice, abdominal pain, and acute pancreatitis, which make exclusion of pancreatic cancer necessary before the diagnosis of AIP. However, unlike pancreatic malignancies, AIP may respond to therapy with corticosteroids.
Although AIP is primarily a pathologic diagnosis, attempts have been made to clinically diagnose AIP using various clinical criteria. In 2011 an international symposium on AIP yielded the International Consensus Diagnostic Criteria (ICDC), which can be used to classify AIP as type 1, type 2, or AIP–Not Otherwise Specified. This article discusses clinical, pathologic, and serologic features of AIP with mention of the various diagnostic guidelines that have been used to diagnose AIP, with a special focus on ICDC. Management options are also discussed.
Clinical Characteristics
Patients with type I AIP typically present at an older age (on average 16 years older) than patients presenting with type 2 AIP. Patients with either type of AIP commonly present with obstructive jaundice, abdominal pain, and/or biochemical evidence of pancreatitis. The study of a large cohort of 731 patients found that obstructive jaundice was the presenting symptom in 75% of patients with type 1 AIP compared with abdominal pain being the most common presentation in 68% of patients with type 2 AIP. The obstructive jaundice may be related to pancreatic swelling and compression of the biliary tree, or be caused by proximal extrahepatic and intrahepatic duct stricture, which can be part of an associated IAC. The abdominal pain is typically mild and may or may not be associated with documented attacks of acute pancreatitis. AIP is not a common cause for idiopathic recurrent pancreatitis. Increases in IgG4 levels are typically seen more often with type 1 AIP, with the degree of increase in IgG4 level necessary to satisfy level 1 evidence for the diagnosis of type 1 AIP greater than twice the upper limit of normal. A level less than twice the upper limit of normal is consistent with level 2 evidence per ICDC guidelines. Imaging findings range from either diffuse or focal pancreatic involvement, often with evidence of other organ involvement (OOI), including hilar lymphadenopathy, extrapancreatic biliary duct involvement, or renal masses. Type 1 AIP is more likely to have biliary tract disease and a higher rate of relapse compared with type 2 AIP.
Diagnostic Guidelines
Because therapy and prognosis differ greatly between the two diseases, exclusion of pancreatic adenocarcinoma must be confirmed before pursuing AIP as a diagnosis. It is also important to diagnose AIP because treatment may avert the long-term consequences of the disease, in addition to avoiding unnecessary surgery. Numerous guidelines have evolved to reasonably differentiate AIP from pancreatic cancer preoperatively using methods that are noninvasive, such as imaging, serology, and trial with steroid therapy. These clinical guidelines include the Japanese Pancreas Society (JPS; JPS-2006, JPS-2011 ), Korean Criteria, Asian Criteria, HISORt (histology, imaging, serology, OOI, response to corticosteroids), and most recently the ICDC.
The Japanese Pancreas Society guidelines
The JPS originally published diagnostic guidelines in 2002, which have since been revised in 2006 and 2011. With JPS-2006, the diagnosis of AIP can be made by 2 possible methods, both requiring imaging. With the addition of serology (showing increased levels of gamma globulins or immunoglobulins) or histology (showing LPSP), diagnosis can be confirmed.
In 2011 these guidelines were revised to yield JPS-2011 to provide more avenues for reaching a diagnosis. The main difference between JPS-2011 and any other diagnostic guidelines is the requirement for diagnostic endoscopic retrograde cholangiopancreatography (ERCP; pancreatogram) in any patient with atypical parenchymal imaging. Without ERCP, the diagnosis of definitive, probable, or possible AIP is impossible with these guidelines. The reason for mandatory ERCP in such cases is to avoid misdiagnosis of pancreatic cancer. For those patients who have typical findings of AIP on parenchymal imaging the diagnosis can be made with the addition of serology, OOI, or histology.
The HISORt guidelines
The HISORt criteria, originating from the Mayo Clinic in 2006, are based on a mnemonic that can be used to recall diagnostic features of AIP. These features include:
- 1.
Histology (H)
- 2.
Imaging (P-Parenchymal; D-Ductal)
- 3.
Serology (S)
- 4.
OOI
- 5.
Response to corticosteroids (Rt)
Considering the available features, patients can meet the diagnosis with evidence of diagnostic histology, typical imaging and serology, or response to corticosteroids.
The Korean and Asian guidelines
The Asian guidelines, formulated in 2008, represent a collaboration between the Japanese and Korean guidelines. Unlike JPS-2006, the Korean guidelines adopted OOI and response to corticosteroid therapy in their guidelines. With the Korean guidelines, OOI or response to steroid therapy can be paired with parenchymal or ductal imaging to diagnose AIP. However, the resulting Asian guidelines concluded that imaging, serology, and histology could be considered as acceptable evidence for diagnosis, and corticosteroid response could be used as an optional collateral piece of data. Hence the definitive diagnosis of AIP is possible with the combination of imaging with either serology or histology only, using these guidelines.
The International Consensus Diagnostic Criteria guidelines
In 2011, an international panel of experts met during the 14th Congress of the International Association of Pancreatology and developed the ICDC ( Tables 1 and 2 ). The significance of these guidelines is they allowed for flexibility in the diagnosis of both subtypes of AIP and can be used in clinical and research practice. Unlike older criteria, they do not require typical pancreatic imaging in order to make the diagnosis, instead providing multiple avenues that can be taken, depending on available data, in order to make definite or probable diagnosis of AIP.
| Diagnosis | Primary Basis for Diagnosis | Imaging Evidence | Collateral Evidence |
|---|---|---|---|
| Definitive type 1 AIP | Histology | Typical/indeterminate | Histologically confirmed LPSP (level 1 H) |
| Imaging | Typical | Any non-D level 1/level 2 | |
| Indeterminate | Two or more from level 1 (+ level 2 D a ) | ||
| Response to steroid | Indeterminate | Level 1 S/OOI + Rt or level 1 D + level 2 S/OOI/H + Rt | |
| Probable type 1 AIP | — | Indeterminate | Level 2 S/OOI/H + Rt |
| Criterion | Level 1 | Level 2 | |
|---|---|---|---|
| P | Parenchymal imaging | Typical: diffuse enlargement with delayed enhancement (sometimes associated with rimlike enhancement) | Indeterminate (including atypical b ): segmental/focal enlargement with delayed enhancement |
| D | Ductal imaging (ERP) | Long (>one-third length of the main pancreatic duct) or multiple strictures without marked upstream dilatation | Segmental/focal narrowing without marked upstream dilatation (duct size <5 mm) |
| S | Serology | IgG4 level >2 × upper limit of normal | IgG4 level 1–2 × upper limit of normal |
| OOI | Other organ involvement | a or b | a or b |
|
| ||
| H | Histology of the pancreas |
|
|
| Rt a | Diagnostic steroid trial | ||
| Rapid (≤2 wk) radiologically demonstrable resolution or marked improvement in pancreatic/extrapancreatic manifestations | |||
a Diagnostic steroid trial should be conducted carefully by pancreatologists with caveats (see text) only after negative work-up for cancer, including endoscopic ultrasonography-guided fine-needle aspiration.
b Atypical: AIP cases may show low-density mass, pancreatic ductal dilatation, or distal atrophy. Such atypical imaging findings in patients with obstructive jaundice and/or pancreatic mass are highly suggestive of pancreatic cancer. Such patients should be managed as having pancreatic cancer unless there is strong collateral evidence for AIP, and a thorough work-up for cancer is negative (see algorithm).
c Endoscopic biopsy of duodenal papilla is a useful adjunctive method because ampullae are often involved pathologically in AIP.
Typically, the first step is to evaluate parenchymal imaging and categorize it as either typical (level 1) or atypical (level 2). Typical characteristics on parenchymal imaging include diffuse enlargement with delayed enhancement, sometimes associated with a rimlike enhancement. Segmental enlargement or delayed enhancement meets atypical or level 2 criteria. Depending on which level of evidence is present, the requirements for supporting evidence vary. For example, patients who have typical/level 1 parenchymal imaging on computed tomography (CT) or MRI would then undergo evaluation for OOI and serum measurement of IgG4 level. If either of these criteria meets level 1 or level 2 evidence, then this would be highly suggestive of type 1 AIP. Patients such as this would then be considered for treatment with corticosteroids. In contrast, in patients who have atypical/level 2 parenchymal imaging, if neither OOI nor serology meet typical/level 1 criteria then an endoscopic pancreatogram and pancreatic biopsy are required in order to proceed with the diagnosis of type 1 AIP.
The ICDC use the 5 cardinal features of AIP:
- 1.
Pancreatic imaging of either parenchyma or ducts
- 2.
Serology
- 3.
OOI
- 4.
Histology of the pancreas
- 5.
Response to corticosteroid therapy (Rt)
Details regarding each of these cardinal features as they pertain to ICDC are discussed later.
Pancreatic imaging
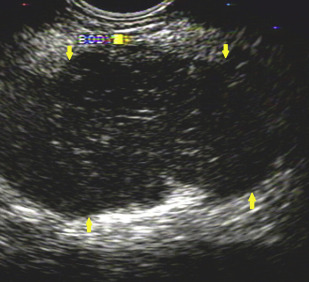
Pancreatic imaging abnormalities are found in up to 85% of patients with AIP. Features suggestive of AIP on CT or MRI are diffuse parenchymal enlargement with delayed enhancement, sometimes associated with rimlike enhancement ( Fig. 1 ). Indeterminate imaging of the pancreas includes segmental or focal enlargement with delayed enhancement. Peripancreatic vascular involvement is rarely described, and can be confused with features of locally advanced malignancy. Endoscopic ultrasonography (EUS) and transabdominal ultrasonography have also been used to evaluate the parenchyma of the pancreas ( Fig. 2 ).
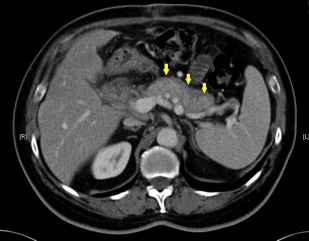
A recent prospective study compared 32 patients with AIP with a control population of patients with pancreatic adenocarcinoma based on CT imaging features. Independently, 3 radiologists read the images and reported common features seen in each disease. The most common findings seen on CT in patients with AIP were common bile duct (CBD) stricture (63%), bile duct wall hyperenhancement (47%), and diffuse parenchymal enlargement (41%). In contrast, in the control population the most common CT imaging features were focal mass (78%) and pancreatic ductal dilatation (69%). In 10 patients with pathologically confirmed AIP, the misdiagnosis of pancreatic adenocarcinoma was made based on radiology primarily because of the presence of a focal mass, which was seen in 9 patients (90%). A similar study compared 101 patients with AIP, pancreatic carcinoma, and control patients without AIP or pancreatic cancer. This study showed that under dual-phase CT scan, the enhancement patterns differ between patients with AIP and those with pancreatic cancer and normal pancreas samples. Features typical for AIP have also been described with the use of multiple detector CT, with the most common features suggestive of AIP being a sausage shape (64%) and low-attenuation halo (59%). Thus, findings on CT that are atypical for AIP should prompt investigation for alternative diagnosis.
ERCP and magnetic resonance cholangiography ductal imaging features, although limited on their own, can provide useful collateral evidence in diagnosing AIP in cases in which parenchymal imaging is noncontributory. Per the ICDC guidelines, typical features on ERCP are long (>one-third the length of the main pancreatic duct) or multiple strictures of the pancreatic duct without marked upstream dilatation ( Fig. 3 ). Indeterminate ERCP features are segmental/focal narrowing of the main pancreatic duct without marked upstream dilatation (duct size <5 mm). Features most agreed on in diagnosing AIP in an international multicenter study were long (one-third the length of the pancreatic duct) stricture, no upstream dilatation, multiple strictures, and side branches arising from a strictured segment. Similarly, a focal stricture of the proximal or distal CBD or irregular narrowing of the intrahepatic ducts can be found. However, pancreatic ductal imaging with ERCP is limited because of the limited role for diagnostic ERCP in clinical practice.
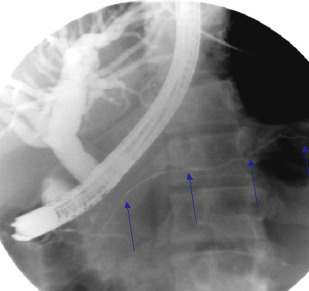
Parenchymal and ductal imaging can serve complimentary roles in establishing a diagnosis of AIP using the ICDC guidelines. Depending on which level of evidence is met, various pathways to diagnosis can be taken. When typical imaging is detected, any nonductal imaging (serology, OOI, corticosteroid therapy) evidence can be used to confirm the diagnosis of definitive AIP and invasive testing with fine-needle aspiration (FNA) biopsy or pancreas histology is not required. However, if atypical imaging is present then the diagnosis hinges on the availability of collateral evidence (see Table 1 ). The key difference that allowed improved utility of ICDC compared with older guidelines was the removal of the requirement of typical imaging findings. Without typical pancreatic imaging, the diagnosis of AIP using the JPS-2006, Korean, and Asian guidelines is not possible.
Serology
IgG4 typically accounts for less than 5% of all the total serum IgG in normal individuals and the level is typically less than 140 mg/dL. Increases in IgG4 or serum antinuclear antibody (ANA) levels can be expected in patients with AIP. Level 1 evidence is defined by at least a 2-fold increase in serum IgG4 level, and increase between 1 and 2 times the upper limit of normal satisfies level 2 evidence (see Table 1 ). This cutoff was chosen because up to 10% of individuals with pancreatic ductal cancer have values less than the 2-fold threshold.
In one study, patients with AIP had high serum concentrations of IgG4, suggesting that increased levels of these immunoglobulins were useful in evaluating patients suspected of having AIP. By evaluating patients with AIP and comparing them with age- matched and sex-matched patients who were normal, the median IgG4 level in patients with AIP was 663 mg/dL compared with 51 mg/dL in normal patients. In a separate cohort, 45 patients with AIP had a mean serum IgG4 level of 550 mg/dL compared with 69.5 mg/dL among 135 patients with pancreatic cancer. Furthermore, serum IgG4 levels were increased in 13 of 135 (10%) the patients with pancreatic cancer but only 1% of this group had IgG4 levels greater than 280 mg/dL, compared with 53% of the AIP subset. An IgG4 level greater than 280 mg/dL corresponds with a sensitivity and specificity of 53% and 99%, respectively. Comparatively, an IgG4 level greater than 140 mg/dl has sensitivity and specificity of 76% and 93%, respectively.
However, increases in IgG4 titers are not specific to AIP and can also be seen in pancreatic cancer. Therefore, reliance on serology alone is an unwise practice and the presence of serology data should be used in tandem with other clinical and diagnostic features. However, there may be a role for IgG4 in monitoring response to medical treatment. Among 12 patients with sclerosing pancreatitis (AIP), serum IgG4 values were measured before corticosteroid therapy and after 4 weeks of therapy. Before therapy, the average IgG4 value in this group was 742 mg/dL compared with 223 mg/dL after 4 weeks, suggesting its role in monitoring treatment response. Antibodies measured in patients with AIP, including anti–plasminogen-binding protein carbonic anhydrase II antigen and lactoferrin, do not have sufficient sensitivity and specificity to separate out AIP from pancreatic malignancy.
Other organ involvement
Given the myriad of extrapancreatic manifestations possible in AIP, it has been proposed that AIP is part of a systemic process caused by IgG4-related disease. The extrapancreatic sites that are commonly involved include the biliary tree, lacrimal and salivary glands, as well as the kidneys, retroperitoneum, pituitary, and prostate. In type 1 AIP, OOI can be detected on radiology or histology. Radiologic evidence showing segmental/multiple proximal (hilar/intrahepatic) or proximal and distal bile duct stricture, retroperitoneal fibrosis, symmetrically enlarged salivary or lachrymal glands, or evidence of renal involvement can all be used to meet either level of evidence for type 1 AIP. Extrapancreatic organs showing evidence of marked lymphoplasmacytic infiltration with fibrosis and without granulocytic infiltration, with storiform fibrosis, obliterative phlebitis, or abundant (>10 cells/HPF) IgG4-positive cells can be used to show histologic OOI.
Initially, AIP was thought to be associated with primary sclerosing cholangitis (PSC); however, the intrahepatic and extrahepatic biliary tract abnormalities with stricture seen in AIP are most often related to an IAC, often with associated increases of serum IgG4 levels. PSC biliary strictures are typically bandlike with a beaded or pruned appearance, whereas IACs are typically segmental, long strictures with a prestenotic dilatation, and are more commonly seen in the distal CBD. AIP-related biliary strictures are more likely to respond to steroids. This distinction from PSC has been confirmed by a pathology study of gallbladders from patients with AIP, PSC, and pancreatic carcinoma. There were dense extramural infiltrates in 41% of gallbladders of patients with AIP, compared with only 4% of patients with pancreatic carcinoma and 0% in patients with PSC. In addition, specimens from patients with AIP showed a higher IgG4/IgG ratio compared with PSC and pancreatic carcinoma counterparts.
Bowel involvement is primarily associated with type 2 AIP; however, there are reports of patients with type 1 AIP concurrently diagnosed with inflammatory bowel disease. According to a multicenter international study that compared 204 patients with type 1 AIP and 64 patients with type 2 AIP, patients with type 2 AIP were more likely to have concurrent inflammatory bowel disease, specifically ulcerative colitis: 16% versus 1%, respectively.
Histology
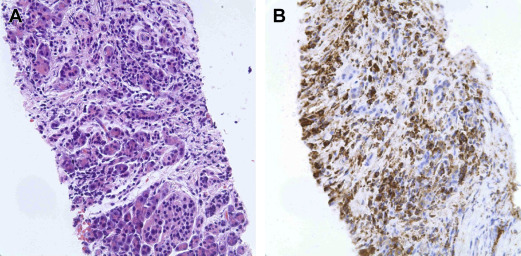
The characteristic histologic feature of type 1 AIP is LPSP, whereas type 2 AIP, also known as idiopathic duct-centric pancreatitis (IDCP), is more classically associated with GELs with or without granulocytic acinar inflammation, along with absent (0–10 cells/HPF) IgG4-positive cells ( Fig. 4 ). However, GELs are not specific for type 2 AIP, and may be found in 27% of type 1 AIP cases.
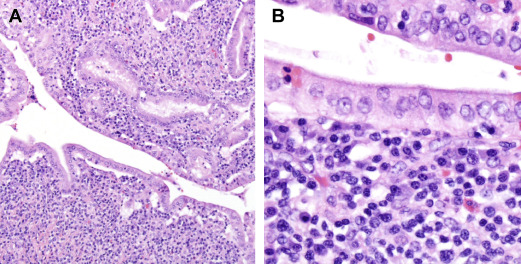
In addition to the histologic evidence of periductal lymphoplasmacytic infiltrate without granulocytic infiltration, at least 2 additional histologic features are required in order to meet level 1 evidence for type 1 AIP. Features that may be combined to make a diagnosis include obliterative phlebitis, storiform fibrosis, and abundant (>10 cells/HPF) IgG4-positive cells. The presence of just 1 additional feature meets the criteria for level 2 evidence.
Unlike type 1 AIP, type 2 AIP requires tissue to confirm the diagnosis per the ICDC guidelines. Lack of association of IDCP with increased levels of IgG4 and OOI requires tissue in order to avoid the misdiagnosis of pancreatic malignancy as AIP. However, obtaining a histologic diagnosis in the absence of surgical resection is challenging. There is too little experience with either EUS-guided or radiology-guided FNA or core biopsies of pancreas to obtain sufficient cytologic or histologic tissue to make a diagnosis ( Fig. 5 ). The additional immunostaining for IgG4 in extrapancreatic tissue may also support the diagnosis of AIP.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



