Fig. 1
Schematic view of the hydraulic occluder used for induction of aortic occlusion during MRI . 1 = Indistensible extension tube, 2 = sutures, 3 = distensible silicone tube, 4 = aorta. A water-filled syringe, connected to the indistensible extension tube, is used to create a hydraulic pressure, which leads to an inflation of the distensible tube. This causes a compression of the aorta and restricts the blood flow
6.
Interferometric temperature measurement system (ACS-P4-N-62SC, Opsens, Quebec City, Canada), including a fiber-optical temperature probe (OTP-M, AccuSens, Opsens, Quebec City, Canada). If you use a GaAs crystal-based system instead be aware of the offset caused by the magnetic field (approx. 4.7 °C at 9.4 T).
7.
For blood pressure measurement: (1) a pressure transducer (DT-XX, Viggo-Spectramed, Swindon, UK) connected to an amplifier (TAM-A Plugsys Transducer; Hugo Sachs Elektronik–Harvard Apparatus GmbH, Mach-Hugstetten, Germany); (2) the femoral artery catheter was designed and made in our laboratory using Portex Tubing (polythene). It must be longer than 1 m to allow placing the pressure transducer well outside the bore of the MR scanner.
8.
For assessment of total renal blood flow: a perivascular flow probe (MV2PSB-MRI ; Transonic Systems, Ithaca, NY, USA) connected to a perivascular flow module (TS420; Transonic Systems).
9.
Combined optical Laser-Doppler-Flux and pO2 probes (pO2 E-Series Sensor; Oxford Optronix) for measurements of local tissue oxygenation and local tissue perfusion . The probes are attached to an OxyLite/OxyFlo™ apparatus (Oxford Optronix, Oxford, UK).
10.
Laboratory power supply (e.g., Model 3200, Statron VEB, Fuerstenwalde, Germany) for creating voltage signal markers (by manual on/off switching) during the experiments. Voltage is set to 5 V and treated as a logical TTL-like signal. This will be referred to as TTL switch in the following.
11.
For continuous logging of the signals from the probes for arterial blood pressure, renal blood flow, cortical and medullary pO2 and flux, together with that from the TTL switch their analogue outputs must be digitized and recorded. An analogue-digital converter (DT 9800-16SE-BNC, Data Translation GmbH, Bietigheim-Bissingen, Germany) permits connection to the USB port of a PC. A dedicated data acquisition software (HAEMODYN™, Hugo Sachs Elektronik–Harvard Apparatus GmbH, March-Hugstetten, Germany) allows calibration of the probe signals and their continuous recording.
12.
For fixation and stabilization of the probes and for the safe transfer of the animal to the scanner, a custom-made portable animal holder (see Fig. 2) must be used, in addition to the MRI animal holder (listed in Subheading 2.3). It was designed and built in our laboratory using 3D CAD (Autodesk Inventor 2012; Autodesk, San Rafael, CA, USA) and rapid prototyping (BST 1200es; Alphacam GmbH, Schorndorf, Germany). The holder must meet the geometry of the MR setup; it has a half-pipe shape with a section of reduced diameter to allow for the four-element surface RF coil to be placed beneath. A mark on the holder indicates the center of the RF coil. A bridge-like construction, positioned at the end of the hind paws of the rat, enables fixation of all leads that connect the physiological probes with the equipment positioned outside the MR scanner room. The portable rigid animal holder in conjunction with the adjustable cable support enables safe transport of the animal to the MR scanner.


Fig. 2
Custom-made portable animal holder. A bridge-like construction (adjustable in height and distance to the animal), positioned at the end of the hind paws of the rat, enables fixation of all leads that connect the physiological probes with the equipment positioned outside the MR scanner room. The frontal part has a section with reduced outer diameter, which leaves a gap to the half-pipe-shaped animal holder of the MR system. Into this gap fits the surface RF coil, which should not be placed exactly horizontally on the holder, but be rotated by 20°–30° towards the side with the kidney of interest (here: left kidney, right side of holder in supine position of animal)
13.
Patches of Dacron gauze (Woven Mesh Spacer; Merck Millipore, Billerica, MA, USA).
14.
Histoacryl™ glue (Braun Surgical GmbH, Melsungen, Germany).
15.
Medical sticky tape.
16.
Silicone elastomer tubes (Silikonkautschuk).
2.3 Magnetic Resonance Imaging
Magnetic resonance imaging (MRI ) requires access to an ultra-high-field MRI system including suitable accessories for the MR acquisition (radiofrequency antennas), positioning, anesthesia, warming, and monitoring of physiological parameters, and trained personnel for operating the MRI system.
Due to the small size of rats in comparison with humans a much higher spatial resolution is required to depict the kidney with adequate detail. This in turn demands a high signal-to-noise ratio (SNR), which must be achieved by use of tailored MR equipment.
1.
MR system: a dedicated small animal MR system with a magnetic field strength of 7 T and higher is recommended. Here we describe the use of a 9.4 T 20 cm bore system (Biospec™ 94/20, Bruker Biospin) equipped with a gradient system with integrated shim set (B-GA12S2, Bruker Biospin; gradient amplitude 440 mT/m, max. slew rate 3440 T/m/s).
2.
Radiofrequency (RF) coils: use RF coils (antennas for RF transmission and reception) suitable for abdominal imaging, such as a transmit/receive rat body volume coil (72 mm inner diameter, quadrature; Bruker Biospin, Ettlingen, Germany) or preferably a transmit only rat body volume coil (72 mm inner diameter, linear; model T10325V3, Bruker Biospin) in combination with a receive only rat heart coil array (curved, 2 × 2 elements; model T12814V3, Bruker Biospin). Use of the latter coil setup is assumed here, as it allows for much higher spatial resolution due to its superior SNR when compared with the transmit/receive volume coil.
3.
Animal holder: an MRI animal holder (here model T11739, Bruker Biospin) designed for the size of the animals and the geometry of the RF coils is provided by the MR system/RF coil manufacturer (see Note 1 ).
4.
Gases: O2, N2, and compressed air, as well as a gas-mixing system (FMI Föhr Medical Instruments GmbH, Seeheim-Ober Beerbach, Germany) to achieve required changes in the oxygen fraction of inspired gas mixture (FiO2). The following gas mixtures are required during the experiment: (1) for hypoxia —10 % O2/90 % N2; (2) for hyperoxia —100 % O2; (3) for normoxia—21 % O2 (air).
5.
Device for FiO2 monitoring in gas mixtures: for example Capnomac AGM-103 (Datex GE, Chalfont St Giles, UK).
6.
Device for warming the animal: use a circulating warm water-based heating system, consisting of a flexible rubber blanket with integrated tubing (part no. T10964, Bruker Biospin) connected to a conventional warm water bath (SC100-A10, ThermoFisher, Dreieich, Germany). For alternative coil setups water pipes may be built into the animal holder.
7.
Monitoring of physiological parameters: for monitoring of respiration and core body temperature throughout the entire MR experiment use a small animal monitoring system (Model 1025, Small Animal Instruments, Inc., Stony Brook, NY, USA), including a rectal temperature probe and pneumatic pillow.
8.
Data analysis: quantitative analysis of the data requires a personal computer and MATLAB software (R13 or higher; The Mathworks, Natick, MA, USA), ImageJ (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2014) including the BrukerOpener plugin and NIfTi Input/Output plugin, FSL (Analysis Group, FMRIB, Oxford, UK, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) or a similar software development environment. Analysis steps described in Subheading 3 can be performed manually by using the functions provided by the software development environment. Most of these steps benefit from (semi-)automation by creating software programs/macros—these steps are indicated by the computer symbol ( ).
).
 ).
).3 Methods
Figure 3 illustrates the work flow, including preparations, experimental procedures, and data analyses. Steps in this work flow chart whose name starts with a number (e.g., 3.1 Preparation of MRI ) are described in the corresponding method sections with the same name.
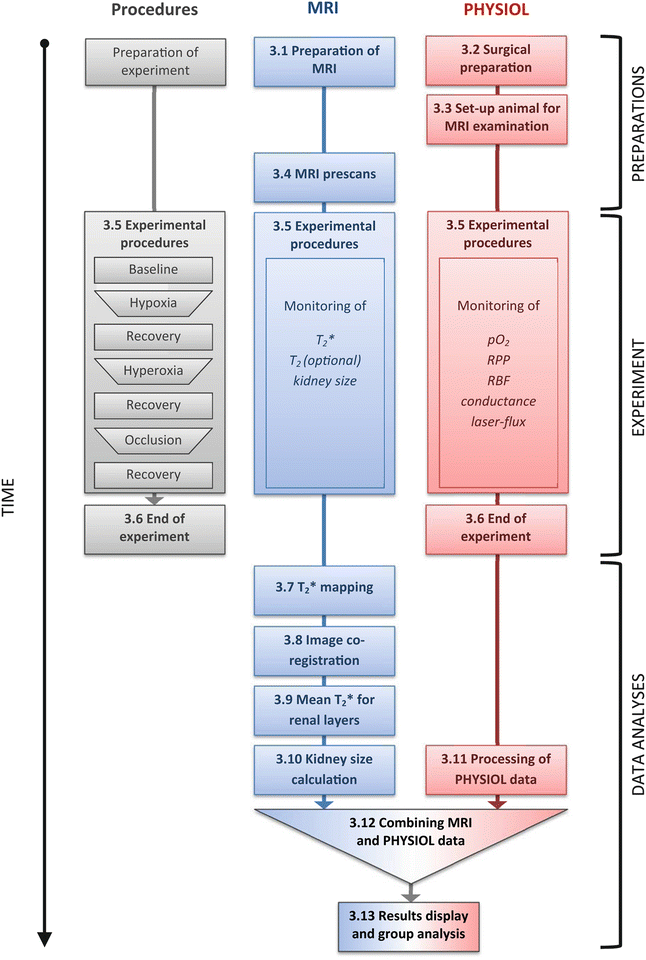

Fig. 3
Illustration of the work flow, including preparations, experimental procedures, and data analyses. Steps in this work flow chart whose name starts with a number (e.g., 3.1 Preparation of MRI ) are described in the corresponding method sections with the same name. pO 2 partial pressure of oxygen, RPP renal perfusion pressure, RBF renal blood flow
3.1 Preparation of MRI
1.
Start the ParaVision™ 5 software and —only before the very first experiment—create and store the following MR protocols.
(a)
Protocol_TriPilot (pilot scan): conventional FLASH pilot with seven slices in each direction (axial, coronal, sagittal).
(b)
Protocol_T2axl (axial pilot scan): RARE sequence, repetition time (TR) = 560 ms, effective echo time (TE) = 24 ms, RARE factor 4, averages = 4. Define as geometry an axial field of view (FOV) = 70 × 52 mm2, matrix size (MTX) = 172 × 128, eight slices with a thickness of 1.0 mm and distance of 2.2 mm, and an acquisition time of approximately a minute. Respiration trigger on (per phase step), flip-back on, fat saturation on.
(c)
Protocol_T2corsag (coronal/sagittal pilot scan): like Protocol_T2axl, but only one slice in coronal orientation.
(d)
Protocol_PRESSvoxel (shim voxel): conventional PRESS protocol, with a voxel size of = 9 × 12 × 22 mm3.
(e)
Protocol_MGE (T 2* mapping): multigradient echo (MGE) sequence, TR = 50 ms, echo times = 10, first echo = 1.43 ms, echo spacing 2.14 ms, averages = 4. Define as geometry a coronal oblique image slice with an FOV = (38.2 × 50.3) mm2, MTX = 169 × 113 zero-filled to 169 × 215, and a slice thickness of 1.4 mm. Respiration trigger on (per slice), fat saturation on. See Note 2 for the optional Protocol_MSME (T 2 mapping).
(f)
Protocol_TOF (angiography): FLASH sequence, TR = 11 ms, TE = 3 ms, flip angle = 80°, spatial in plane resolution of 200 × 268 μm2, with 15 slices of 1.0 mm thickness.
2.
Switch on the gradient amplifiers of the MR system, which will also power on the automatic animal positioning system AutoPac™.
3.
Install the transmit/receive rat body volume coil in the magnet bore.
4.
Connect the animal holder to the animal positioning system (AutoPac™).
5.
Install the rat heart coil array RF coil including its preamplifier on the animal bed.
6.
Attach the face mask unit (commercial or custom-made, as described in Note 1 ) to the animal holder and connect it to the inspiratory gas providing system (luer tubing).
7.
Place the flexible rubber mat of the warm water-based heating system on top of the rat heart coil array and connect it to the warm water circulation.
9.
Attach the rectal temperature probe and pneumatic pillow to the small animal monitoring system and place the probes on the animal bed, approximately at the later abdominal position of the rat.
3.2 Surgical Preparation
Surgery must be performed parallel to MRI preparation outside the MR scanner room (in a neighboring preparation room) for safety reasons.
1.
Anesthetize the animal by intraperitoneal injection of urethane (20 % solution, 6 mL/kg body mass) (see Note 5 ).
2.
After reaching the required depth of anesthesia (determined by specific physiological signs such as muscle relaxation degree, absence of the paw withdrawal reflex, absence of the swallowing reflex, whisker movements, etc.), carefully shave the coat in the abdominal area of the rat (hair clipper Aesculap Elektra II GH2, Aesculap AG, Tuttlingen, Germany).
3.
Place the rat in supine position on a warmed-up (39 °C) temperature-controlled operating table and fix the paws of the animal to the table by means of sticky tapes.
4.
Make an incision in the left inguinal area (approximate 12 mm) along the natural angle of the hind leg.
5.
Bluntly dissect the connective tissue until the femoral artery and vein are exposed.
6.
Gently separate the nerve. Do not cut or damage the nerve.
7.
Using fine tip forceps separate the vein from the artery, trying to expose an approximately 7–8 mm length fragment.
8.
Place three pieces of 4.0 threads under the femoral artery: the first piece towards the leg, the second towards the body, and the third one between them.
9.
Pull the first thread towards the leg and tie this into a triple knot.
10.
Prepare loose knots on the remaining two.
11.
Pull the second piece of thread towards the body to stop the blood flow into the femoral artery.
12.
Using fine tip scissors make an incision in the exposed segment of the femoral artery. Fill the catheter with saline.
13.
Grasp the catheter with the forceps and gently push through the incision into the lumen of the femoral artery.
14.
Tie the third knot, relax the stretched second thread, and push the catheter deeper (≈10 mm) into the artery.
15.
Rinse the catheter carefully with saline, making sure that it is patent.
16.
Tie the prepared loose knot of the second thread.
17.
Start the monitoring of arterial blood pressure.
18.
Open the abdominal cavity by a midventral incision (4–5 cm). Carefully dissect the aorta directly above both renal arteries from the surrounding tissues.
20.
Using fine tip forceps carefully separate the renal artery from the vein, trying to expose an approximately 6–7 mm length fragment. Do not cut or damage the nerves.
21.
Transfer the animal onto the portable animal holder such that the kidney is aligned with the mark on the holder that indicates the center of the RF coil.
24.
Remove the customary Luer-Lock connectors of the laser-flux-pO2 probes and fix the fiber glass cores with customary silicone tubing by means of medical sticky tape. Attach tailored patches of gauze to the end of the silicone tubing (see Fig. 4).
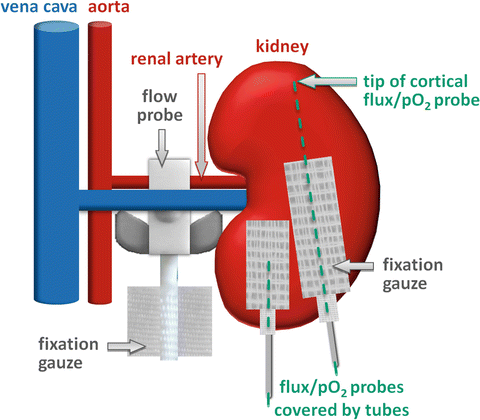

Fig. 4
Schema illustrating the position of the perivascular probe for total renal blood flow and the cortical and medullary flux/pO2 probes together with measures taken to fix probes at their respective positions
25.
Measure the diameter between the caudal and the cranial extremities of the left kidney with a caliper gauge. Based on this measurement the cortical laser-flux-pO2 probe must be carefully prepared so that the distance between the insertion point and the tip exactly matches the individual kidney’s diameter minus 1.5 mm.
26.
Advance the cortical laser-flux-pO2 probe meticulously from the caudal extremity of the kidney along the caudo-cranial axis (see Fig. 4).
27.
To prevent cranio-caudal displacement the patch of gauze fixed to the silicon tubing of the probe must be glued to the capsule of kidney’s ventral surface by means of the histoacryl™ glue.
28.
Prepare the medullary laser-flux-pO2 probe so that the distance between the insertion point and the tip is exactly matches 3–4 mm (see Fig. 4).
29.
Carefully advance the medullary laser-flux-pO2 probe from the caudal extremity of the kidney along the caudo-cranial axis (see Fig. 4).
30.
Glue the patch of gauze fixed to the silicon tubing of the probe to the capsule of kidney’s ventral surface histoacryl™ glue.
31.
To prevent displacement carefully fix the two probes’ tubing to the retroperitoneal muscles by sutures.
33.
Connect the probes with OxyLite/OxyFlo™ apparatus and start the monitoring of tissue pO2 and laser-Doppler-flux.
34.
Place a fiber-optical temperature probe in close proximity to the kidney, in order to monitor the temperature of the kidney throughout the investigation.
35.
Mark the localization of the investigated kidney’s upper and lower pole on the skin of the abdomen using a pen (see also step 0).
36.
Fill the abdominal cavity with warm saline (37 °C). For replenishment of abdominal saline a catheter must be placed in the abdominal cavity.
37.
Fix all the extensions (temperature probe, Transonic probe, laser-flux-pO2 probes, aortic occluder, and abdominal flushing catheter) to the bridge of the portable animal holder (cable support that prevents the disarrangement of the probes and enables a safe transport of the animal to the MR scanner).
38.
Pass the extensions for aortic occluder, for abdominal flushing, as well as temperature and Transonic probe, through the caudal cutting edge of the median abdominal incision. The extensions of the laser-flux-pO2 probes must be led through the abdominal wall using a small incision in the left inguinal region.
39.
Close the abdominal cavity by continuous suture.
40.
Check that the kidney (pen markings on skin) is still aligned with the mark on the portable animal holder that indicates the center of the RF coil—if necessary carefully correct the animal’s position. This is essential for optimal positioning of the rat in the MR scanner (i.e., optimal position of the rat’s kidney relative to the MR coil).
41.
Re-start the HAEMODYN™ software (to start a new data file) and check the quality of all physiological signals, i.e., renal perfusion pressure (RPP), renal blood flow (RBF), cortical and medullary Laser-flux, cortical and medullary pO2.
3.3 Set Up Animal for MRI Examination
2.
Position portable animal bed with the rat on the MRI animal bed.
3.




Place a respiratory mask loosely around the muzzle of the spontaneously breathing rat. Open air supply to a rate of 1000 mL/min.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







