Appendix
INTRODUCTION
Over a century ago, two major things put the appendix in the public eye. The first was on the medical front where the appendix was firmly on the map as a cause of disease, culminating in a variety of books written on the appendix, its diseases, and its surgical removal in the decades either side of 1900. The ability to safely remove appendices is attributable to the development of anesthesia and subsequently infection control pioneered by the development of the germ theory by Louis Pasteur, and its subsequent application to surgical sterility by Joseph Lister. Probably the largest treatise on the Appendix was the 800 page book with 400 illustrations by Howard A. Kelly and Elizabeth Hurdon published in 1903, but now available online. This book appears to document the first known recorded case of a disease of the appendix, attributed to Mestivier in 1759 in France, where much of the medical writing was done. The first recorded case of perforated appendix outside of France was attributed to Parkinson in the United Kingdom in 1812. Interestingly, the “big four” infectious causes of appendicitis were attributed to tuberculosis, typhoid, ameba, and actinomycosis.
The social acceptance of appendicitis occurred in 1902 with the delay in the coronation of King Edward VII in England in 1902 because of his appendiceal abscess, after which it became fashionable and very acceptable to have appendicitis!
Appendectomy (appendicectomy) remains one of the most commonly performed operations even though the means of removing it is dynamic and—can be open, laparoscopic, or even transgastric or transvaginal. It has been estimated that more than 280,000 appendectomies are performed in the United States every year, and appendiceal tumors are noted in 0.9% to 1.4% of these.1, 2 However, in practice, pathology that affects subsequent patient management is found in only about 1% to 2% of appendices.3, 4 Diseases such as endometriosis, sometimes worms, and occasionally intraperitoneal tumors found in resected appendices account for some of the others. Despite the application of laparoscopic appendectomies and early use of imaging in the diagnosis of appendicitis, over the last three decades the rates of perforated appendicitis have stayed similar, in contrast to nonperforated appendices.1
Despite the large number of resected appendices, there remains a surprisingly large number of problems regarding the pathology of the appendix and its implication for management and prognosis. Problems still include the causes of acute appendicitis and why it has been so difficult to identify major causes, especially those associated with infection, which, other than obstructive causes, have to be the primary cause of acute appendicitis. Conversely, the frequency with which normal resected appendices can be expected to be found in patients whose appendix is removed for symptoms that might be attributed to the appendix seems to be less of an issue, largely because in many centers, imaging has helped hugely in preoperative diagnosis, so that unexpected appendiceal disease is relatively uncommon.
An infrequent but troublesome issue continues to be that of terminology for appendiceal tumors. A major change since the previous edition is that the proximal margin (base) of the appendix is now usually both identified and sectioned, so that adequacy of resection can be evaluated, if a tumor is identified incidentally. The greatest problem is the finding of a tumor either grossly or incidentally on microscopy, its clinical implications, and how it should be managed.
Problems occur in three major areas
1. mucinous tumors and whether all of them are neoplastic, and the risk of pseudomyxoma if they have perforated;
2. the tumors called goblet cell carcinoids (microglandular carcinoma), whether they are really carcinoids,
carcinomas, or a spectrum that can include both, and how they should be managed, especially in terms of additional surgical treatment; and
carcinomas, or a spectrum that can include both, and how they should be managed, especially in terms of additional surgical treatment; and
3. the circumstances under which serious consideration should be given to right hemicolectomy following appendectomy.
It should also be noted that polyps at the appendiceal orifice cannot be removed by laparoscopic appendectomy as the margin of excision is distal to the polyp. Gastroenterologists sending such patients for surgical excision, and surgeons carrying out appendectomy for appendiceal tumors, need to be aware of this limitation. The appendiceal orifice, and therefore at least a cuff of cecum need to be removed.
FUNCTION OF THE APPENDIX
This has been questioned, and it is possible that it acts as a reservoir to repopulate the large bowel flora. The presence of an appendix may reduce recurrences of infections such as that of Clostridium difficile, although it also seems possible that once C. difficile is in the appendix it may act as a reservoir. Lower rates of appendectomy have been found in patients having had C. difficile infections.5, 6, 7, 8 Conversely it has also been recognized that ulcerative colitis is often accompanied by appendiceal disease, which may be repopulating the large bowel with inappropriate flora.9 Some data suggest that appendectomy both prevents ulcerative colitis, or if carried out during the course of the disease, may reduce the severity of recurrences, and the amount of medication required.10 Indeed trials are underway to determine if appendectomy can be used therapeutically in ulcerative colitis.
It has been suggested that the appendix may be the bursa equivalent governing B-cell regulation; however, patients with appendiceal agenesis have yet to be described with immune deficits or a predisposition to tumors.
THE NORMAL APPENDIX
Normal Anatomy, Development, and Gross Appearance
The appendix and cecum are first apparent as an outgrowth from the primitive midgut at the end of the fifth gestational week. The proximal quarter of this outgrowth forms the cecum, and the distal threequarters is destined to become the appendix, rapidly tapering down in the process. Toward the end of intrauterine life the lateral wall of the cecum grows at a greater rate than the medial wall, so that the appendiceal orifice appears to migrate up the medial cecal wall and ultimately comes to lie immediately beneath the ileocecal valve.
The appendix therefore arises from the medial wall of the cecum and averages 6 to 7 cm in length in the adult (Fig. 15-1). In infants it may be as small as 2 cm, but occasionally it reaches 15 cm or more in length. The current record of longest appendix is 26 cm (Guinness Book of Records). In the adult, the average diameter is approximately 0.7 cm. It is easily found by following the taeniae coli of the large bowel, as all three terminate together at the base of the appendix, where they unite to invest the appendix completely with a full longitudinal muscle coat. In the adult the appendiceal orifice is approximately 2.5 cm below the ileocecal valve (Fig. 15-1), and from here its position may vary. Most commonly, it lies posterior to the cecum or ascending colon; the next most frequent site is overhanging the pelvic brim. In this location it may directly impinge on the bladder, with resulting dysuria if inflamed. It may also lie at the side of the cecum, either in front of or behind the terminal ileum, or it may lie directly on the psoas muscle. The appendix may rarely be found in a subhepatic location, primarily because incomplete rotation of the bowel results in failure of descent of the cecum. Both the cecum and the appendix may therefore be in a subhepatic location. In patients with situs inversus the appendix is found in the left iliac fossa.
The appendiceal orifice has no uniform shape when viewed from within the cecum; frequently, a small flap of mucosa may partially overhang its orifice (Fig. 15-1). The mesoappendix consists predominantly of fat but contains the appendiceal blood vessels and occasionally small lymph nodes. Its length tends to
govern the mobility of the appendix and therefore, to a certain extent, its final position. The vascular supply of the appendix is from the posterior cecal branch of the ileocolic artery, itself a terminal branch of the superior mesenteric artery. The venous return is into the superior mesenteric vein and then into the portal vein. Occasional lymph nodes may be present in the mesoappendix which then drain to the pericolic and superior mesenteric nodes.
govern the mobility of the appendix and therefore, to a certain extent, its final position. The vascular supply of the appendix is from the posterior cecal branch of the ileocolic artery, itself a terminal branch of the superior mesenteric artery. The venous return is into the superior mesenteric vein and then into the portal vein. Occasional lymph nodes may be present in the mesoappendix which then drain to the pericolic and superior mesenteric nodes.
Histology
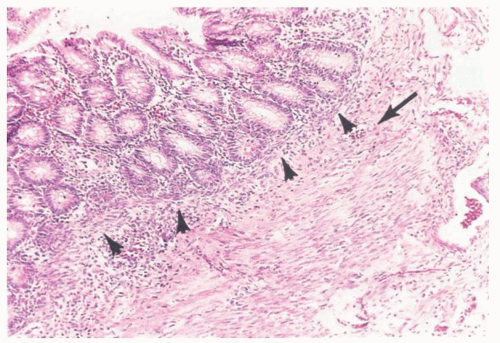
The mucosa of the appendix is primarily of the large bowel type, although it has a much heavier lymphoid component. In the neonate, however, there is very little lymphoid tissue and almost no cells in the lamina propria (Fig. 15-2). Within a few months, the amount of lymphoid tissue increases rapidly to form lymphoid nodules with germinal centers, and by puberty these are the dominant features in the appendix (Fig. 15-3). After puberty they gradually regress, and in the elderly they may disappear. It remains questionable as to whether lymphoid tissue can become exuberant enough to cause luminal obstruction with secondary obstructive acute appendicitis, but it seems likely. Interestingly, there are no objective criteria for lymphoid hyperplasia in the appendix (like elsewhere) so it remains a subjective diagnosis. However it is always worth considering infective causes, which in children should include adenovirus infection (see subsequently in this chapter).
The appendiceal epithelium is of the typical large bowel type, consisting primarily of absorptive and goblet cells and occasional endocrine cells, primarily enterochromaffin (EC) cells (Fig. 15-4). Free endocrine cells may also be present in the lamina propria (Fig. 15-5).11 These lamina propria endocrine cells contain serotonin and are intimately associated with nerves; they appear to be far more frequent in countries with a high incidence of acute appendicitis compared with low-incidence countries,12 although it is unclear how this situation relates pathogenetically to acute appendicitis. One study suggests that these cells are lost in infants with Hirschsprung’s disease along
with the nerves that are usually intimately associated with them.13 Surprisingly, Paneth cells are rare in the appendix even though they are not uncommon in the cecum. The epithelial cells immediately over the lymphoid follicles often have fewer goblet cells and enlarged nuclei, and are reminiscent of the microfold cells in the small intestine.
with the nerves that are usually intimately associated with them.13 Surprisingly, Paneth cells are rare in the appendix even though they are not uncommon in the cecum. The epithelial cells immediately over the lymphoid follicles often have fewer goblet cells and enlarged nuclei, and are reminiscent of the microfold cells in the small intestine.
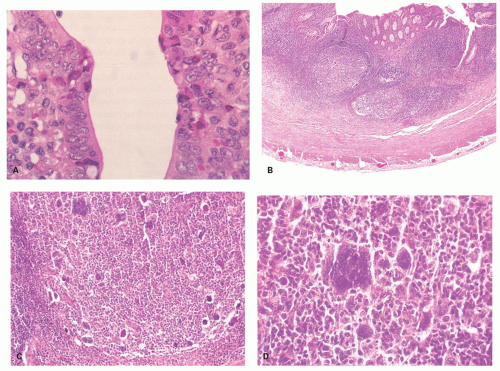
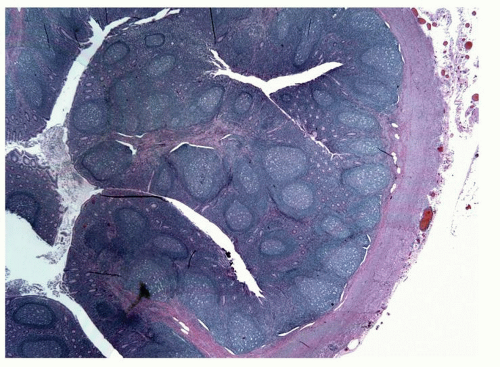 Figure 15-3. Typical nodular lymphoid tissue with well-formed germinal centers having a corona of small lymphocytes polarized toward the lumen. |
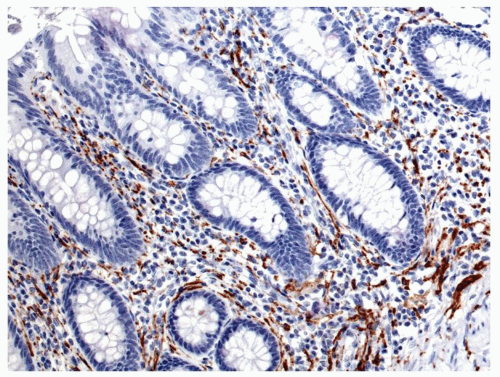
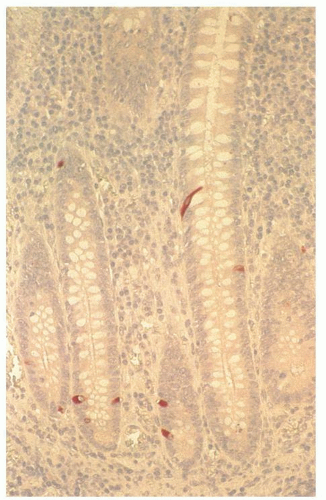 Figure 15-4. Mucosa of normal appendix immunostained with chromogranin A to show the endocrine cells, most of which are enterochromaffin cells. |
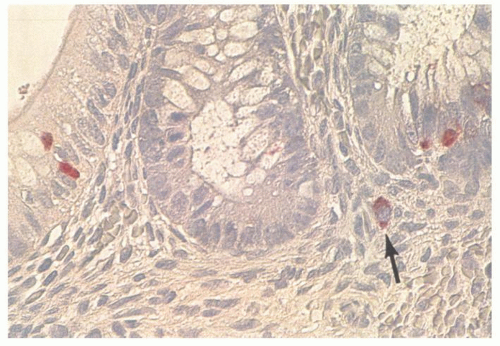 Figure 15-5. Individual free endocrine cell in the lamina propria, as demonstrated by chromogranin A immunoreactivity (arrow). |
The lamina propria in the adult contains a predominance of immunoglobulin A-producing plasma cells. A smattering of other cells are present, including histiocytes, which tend to be relatively superficial, occasional eosinophils. Numerous nerves are also present in the lamina propria (Fig. 15-6), and these include calretinin immunoreactive nerves (Fig. 15-7B).
The muscularis mucosae may be broken regularly by the lymphoid follicles and may virtually disappear in these areas. The submucosa is without fat in the neonate; submucosal fibrosis uniting the muscularis mucosae and propria is therefore normal in children and should not be interpreted as evidence of previous appendicitis (Fig. 15-2).
The muscularis propria contains a complete circular and longitudinal muscle coat. As the latter is formed from fusion of all three taeniae coli, unlike the remainder of the large bowel, it is of uniform thickness throughout.
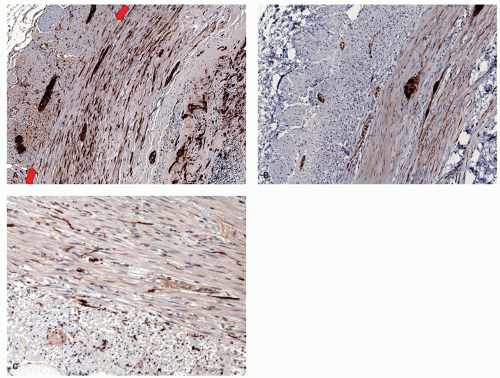
The muscularis propria of the appendix is richly innervated (Fig. 15-7A). The myenteric plexus is unusual in the appendix, and although standard sources indicate it is as elsewhere in the bowel, that is not our experience. The myenteric plexus is sometimes identifiable, but more usually it is rudimentary, with ganglion cells being scattered almost randomly and sometimes diffusely in both the internal circular and longitudinal muscle, rarely being almost in the subserosa (Fig. 15-7A). Many of the neurons and nerves are calretinin immunoreactive (Fig. 15-7B). Further, interstitial cells of Cajal (ICCs) are also diffusely distributed throughout both muscle layers (Fig. 15-7C).14, 15 In right hemicolectomies, especially those carried out for complex masses involving the appendix, the issue may be whether sections come from the large bowel or the adjacent appendix. The distribution of ganglion cells and the myenteric plexus (or lack of it) is an easy way of telling them apart. Individual ganglion cells as well as clusters may be seen elsewhere in the appendix.16 This becomes important in a patient with potential long-segment Hirschsprung’s disease in whom the appendix is removed to see if ganglion cells are present. The atypical location of ganglion cells and lack of plexus may lead to a panicked consideration of conditions such as intestinal neuronal dysplasia and give a false impression of an abnormality in the distribution of ganglion cells rather than recognizing that this is normal. In the neonate, if ganglion cells are being sought as evidence for lack of Hirschsprung’s disease, particularly on frozen section, nucleoli may be difficult to see and cytoplasm may not be well developed. Distinction from prominent endothelium depends on the orientation of the latter toward the lumen and the larger size of the ganglion cell (Fig. 15-8).
ROUTINE PATHOLOGIC EXAMINATION OF THE APPENDIX
Examination of the appendix is primarily aimed at the diagnosis of acute appendicitis and identification of tumors (incidental or otherwise). Extreme care in sectioning of the appendix is needed when the appendix appears distended and a mucinous neoplasm is suspected. Standard practice is to bisect the tip longitudinally for a distance of about 2 cm and then
make transverse sections approximately every centimeter in order to try to detect a fecalith, polyps, or any tumors in the wall. If no abnormality is seen grossly, in addition to a longitudinal section of the tip, two or three random cross-sections are included for histologic examination. Ideally the mesoappendix should be included in its entirety if present along with these sections, when possible. The proximal resected margin should be submitted in all cases to avoid problems regarding adequacy of local excision should an incidental tumor be identified. Clearly this section must be identifiable microscopically. Some ink this margin with a specific color, some make a single lumen to serosal cut so that the section is readily identifiable microscopically, while others put it in a separate cassette.
make transverse sections approximately every centimeter in order to try to detect a fecalith, polyps, or any tumors in the wall. If no abnormality is seen grossly, in addition to a longitudinal section of the tip, two or three random cross-sections are included for histologic examination. Ideally the mesoappendix should be included in its entirety if present along with these sections, when possible. The proximal resected margin should be submitted in all cases to avoid problems regarding adequacy of local excision should an incidental tumor be identified. Clearly this section must be identifiable microscopically. Some ink this margin with a specific color, some make a single lumen to serosal cut so that the section is readily identifiable microscopically, while others put it in a separate cassette.
Unfortunately, making only a few transverse cuts in the appendix rather than slicing it every couple of millimeters severely reduces the likelihood of finding a variety of focal abnormalities, such as diverticula, polyps, and small NETs/carcinoid tumors, simply because no attempt is made to look for them. Although it can be argued that findings of this sort are largely academic, if the appendix is worth examining, then more than a superficial, cursory examination is needed. While only about 1% to 2% of appendices have pathology that modifies subsequent patient management,14, 15 pathology is only recognized by gross examination in about 20% of those patients,17 mainly those with mucinous tumors. Further, although the
mesoappendix is sometimes trimmed, should tumors be found that infiltrate into the mesoappendix, then the transected mesoappendiceal margin becomes a margin of excision. The tumor most likely to involve the mesoappendix is the goblet cell carcinoma (GCC).
mesoappendix is sometimes trimmed, should tumors be found that infiltrate into the mesoappendix, then the transected mesoappendiceal margin becomes a margin of excision. The tumor most likely to involve the mesoappendix is the goblet cell carcinoma (GCC).
Gross Handling of Dilated Appendices
Dilated appendices in which a mucinous tumor is suspected should be handled with care, specifically to avoid problems that can arise in the interpretation of these tumors. The first questions are (i) whether there is mucin on the external surface grossly and (ii) whether the appendix is perforated. Next, if there is still no visible mucin, the body of the still unopened appendix can be gently squeezed to determine whether this causes any luminal contents to extrude from the appendix that might represent a subtle perforation. The objective is not to determine the weakest point in the wall of a nonperforated appendix so gentleness is imperative. If a site is noted, mark or ink that site so that a section can be taken at that site. If there is no evidence of either perforation or external mucin, then the next major issue is that, once the external surface has been entirely inked including any mesoappendix, that when the appendix is finally opened, care is taken to ensure that any luminal content, and especially mucin, is not spilled as it may adhere to the external surface of the appendix. Then when sections are examined, mucin (cellular or acellular) will be visible on the external surface, which causes a huge problem when luminal neoplastic epithelium is present. The question here is whether it is real, with its implied risk of pseudomyxoma peritonei (PMP), or carryover. The purpose of the exercise is therefore to minimize or remove the risk of carryover of mucin onto the external surface. Any potential points or perforations are sectioned.
Ensure that the external surface is dry, and open the appendix over a sink (we prefer opening the tip first and letting internal mucin drain, taking care not to let it contaminate the residual appendix). Then grasp the mesoappendix and make it the most superior part of the specimen to preserve it and open the appendix along the dependent anti-mesoappendiceal margin and allow residual contents to drain, noting their consistency. Examine the opened lumen and wall, and take multiple transverse sections through any lesions, the thinnest part to determine the extent of involvement of the wall if the wall has focal thinner areas, and then random sampling, for example, a block being taken each centimeter, the proximal resection being marked in a way that makes microscopic identification possible (see above) and embedded en face. If the surgeon was alert and aware that a mucocele was present either pre-operatively or intra-operatively, it makes incredibly good sense to take a cuff of cecum with the base of the appendix. By far the most likely problem to arise is involvement of the proximal margin, so this takes care of that eventuality at the time of resection. If not too dilated or large, it may be possible to embed the entire appendix in a reasonable number of blocks, for example, 10 to 20. The advantage of this is with those appendices that have very little epithelium on which to make a diagnosis. It is useful to take all sections in the circumferential plane from the base to tip, including the mesoappendix.
CONGENITAL STRUCTURAL ABNORMALITIES
Positional Abnormalities
The most common structural abnormality of the appendix is its anatomic location. While most are retrocecal or dangling into the pelvis, they can be buried in the cecal wall,18 exist in varying degrees of malrotation, be found higher in the right abdomen, or be even on the left side. But these are all recognizable by following either the terminal ileum into the large bowel (in situs inversus this is reversed) or typically by following the large bowel teniae to where they fuse proximally, which is where the appendix should be located. Problems associated with partial malrotation can be detected this way.
Agenesis. This is a rare condition occurring in <1:10,000 of the population,19 and in about 20% of cases there is associated hypoplasia of the cecum.20, 21 Occasional cases of appendiceal agenesis have been associated with prior maternal thalidomide ingestion.22, 23 There is also a case report of ileal atresia associated with absence of the appendix.24 In Appendiceal hypoplasia, rudimentary appendiceal lymphoid tissue may be present within the cecum.20 Trisomy 18 may be associated with a variety of gastrointestinal (GI) and extra-GI congenital abnormalities, including agenesis of appendix.
Absence of Lymphoid Tissue This is rare but, as expected, occurs in patients with severe immunodeficiency diseases such as thymic alymphoplasia.25
Duplication
Mucosal Duplication Although duplications are usually inferred to mean more than one appendix, there is also a small literature regarding appendices (appendixes is also grammatically acceptable as a plural) with two, and rarely three lumina, each with a complete muscularis mucosae within the same muscularis propria. While one can wonder whether these are really acquired following acute appendicitis with reepithelialization of inflammatory or fistula tract or even
diverticula, there is no good reason why this could not also be genuine. The identification of multiple appendices in the setting of acute appendicitis with an ulcerated mucosa may be difficult or impossible, unless there is clearly (at least) one normal appendix and one inflamed appendix.
diverticula, there is no good reason why this could not also be genuine. The identification of multiple appendices in the setting of acute appendicitis with an ulcerated mucosa may be difficult or impossible, unless there is clearly (at least) one normal appendix and one inflamed appendix.
Table 15-1 Modified Wallbridge Classification of Appendiceal Duplication | |
|---|---|
|
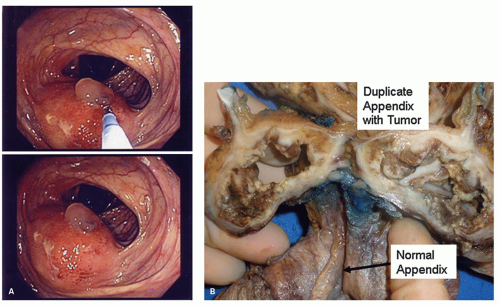
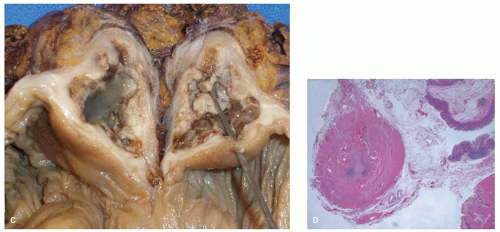
Partial or Complete Duplication Duplication of the appendix is more common than agenesis, with cases of the former regularly appearing in the literature. In the absence of cecal duplication, appendiceal duplication may take several forms. One is two separate complete appendices, usually placed symmetrically in the cecum, one on either side of the ileocecal valve. The least common variant is a rudimentary second appendix arising from the cecum.26 A classification for duplications of the appendix is shown in Table 15-1. Apparent triple appendices have also been documented.27 The Wallbridge classification was originally used but has been modified and forms the basis of all new reports of the condition and its variants.28, 29
Implications The main clinical implication is that patients who have had an appendix removed may still have acute appendicitis in the second appendix, in some ways mimicking appendicitis in an appendiceal stump. Simultaneous appendicitis of more than one appendix is also described, but would be expected if the cause was something that could be predicted to affect both appendices, such as an enteric infection. However all appendices are susceptible to the full range of appendiceal pathology, including neoplasia (Fig. 15-9).
Appendix helicus and horseshoe appendix. This is a peculiar abnormality characterized by a corkscrew configuration of the appendix. It has been described only in association with lumbosacral meningomyelocele.30
Congenital fistula. There is a report of an umbilical defect through which the cecum and appendix had herniated, the latter being intussuscepted but visible externally.33 This was reported as an appendicocutaneous fistula. In another patient the appendix was found in the umbilical stump.34
Patent omphalomesenteric duct involving the vermiform appendix was described in a congenital appendicoumbilical fistula in a neonate: Usually anomalies of the omphalomesenteric duct or urachus result from failure of closure of the umbilical fascial ring. Persistence of the duct may lead to anomalies including umbilical sinus and cyst, Meckel’s diverticulum, or patent omphalomesenteric duct, the latter usually being associated with the ileum, but occasionally with the cecum or appendix.35
Heterotopic tissue. There are case reports of gastrictype mucosa being found in the appendix, one possibly in association with esophageal mucosa. Neither case was associated with peptic ulceration.36, 37 While it is assumed that these conditions are congenital, pyloric metaplasia is relatively frequent as a change in the intestine following ulceration, although much less so in the appendix.
ACQUIRED STRUCTURAL ABNORMALITIES
Diverticula
The vast majority of diverticula of the appendix are presumed to be acquired, that is, they are an outpouching of mucosa and submucosa through the muscularis propria. However up to 10% are thought to be congenital, although the figure is likely closer to 3%38, because they are surrounded by a full muscularis propria.39, 40 These diverticula are said to be single and predominate in males, but in practice they seem to be frequently multiple. They are said to be less common after the third decade of life.39 There is a reported association with Trisomy D syndrome.41
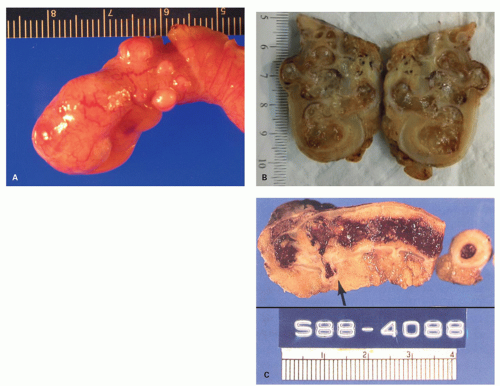
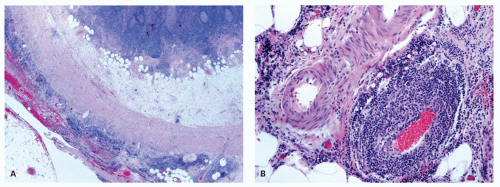
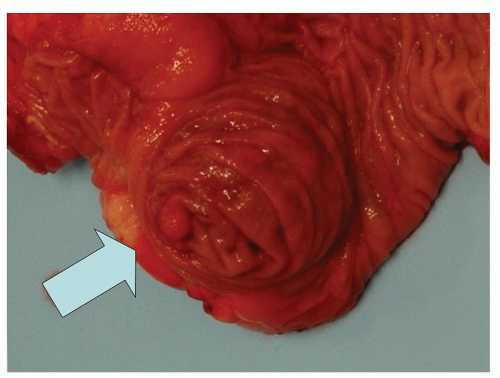
Acquired diverticula are said to occur in up to 2% of resected appendices,39 but their prevalence is likely underestimated because most of them are only a few millimeters in diameter and may also be overlooked if the appendix is not examined carefully or just sliced at 1-cm intervals. In addition, if they are the seat of acute appendicitis (Fig. 15-10), they may give rise to an almost immediate perforation which destroys any evidence of the original diverticulum, although the fistula-like appearance of the inflammation is often a tip-off. A localized focus of acute inflammation and perforation is also highly suspicious when the appendix is not otherwise externally inflamed. When diverticula are present, they are multiple in about half of the cases (Fig. 15-10). They
seem to be relatively evenly distributed between the mesenteric and antimesenteric borders; bearing in mind that there are not gaps between tenia through which they can pass. Occasionally they may be found on both borders; and about one-quarter are present at the tip.40 Finally even if present on histologic sections, they may be regarded as a curiosity or go unrecognized.
seem to be relatively evenly distributed between the mesenteric and antimesenteric borders; bearing in mind that there are not gaps between tenia through which they can pass. Occasionally they may be found on both borders; and about one-quarter are present at the tip.40 Finally even if present on histologic sections, they may be regarded as a curiosity or go unrecognized.
Although the diagnosis of diverticulum of the appendix is straightforward when it penetrates the muscularis propria or when there is an obvious outpouching, sometimes a diverticulum-like appearance is seen limited to the submucosa. While this may represent a forme fruste for pseudodiverticulum, it likely also results from resolved acute appendicitis in which there has been abscess formation into the submucosa with subsequent reepithelialization, a sort of appendicitis cystica profunda.
A variation on this theme is the localized extension of an adenoma into or through the wall of the appendix. Unless this represents a preexisting diverticulum that has been overrun by adenomatous epithelium, most of these may represent adenomas that have eroded through the wall of the appendix (see subsequent discussion).
Complications of appendiceal diverticula are those associated with acute inflammation causing localized perforation and, rarely, hemorrhage, although this may be massive.42 Diverticula are sometimes associated with mucinous neoplasia, but a more difficult issue is when confronted with appendices with acute appendicitis and acute inflammatory perforation that also contain a mucinous tumor, the issue being whether there has been perforation through diverticula with both acute appendiceal diverticulitis and a mucinous tumor (see subsequent discussion).
Diverticula may cause problems because of the rapidity with which clinically these can proceed to perforation, and may also appear as a hole in the appendix with relatively little inflammation elsewhere, other than some serosal exudate. In addition, it is theoretically possible for acute appendiceal diverticulitis to
cause right iliac fossa pain almost as soon as the inflammatory process is through the muscularis mucosae; its significance as a clinical problem is unclear.
cause right iliac fossa pain almost as soon as the inflammatory process is through the muscularis mucosae; its significance as a clinical problem is unclear.
Torsion and Volvulus
Torsion is a twist of less than 360 degrees, and volvulus of more than 360 degrees, so the etiologies of these are similar. In practice both are rare and most reports are associated with tumors, primarily mucinous neoplasms with or without distension, but sometimes because of adhesions due to pathology in other organs (e.g., ovary, tube).43, 44, 45 Dilatation, usually of the distal end of the appendix is followed by rotation immediately proximal to the tumor. This combination results in distal ischemia and hence the clinical presentation. Distal carcinoid tumors can also lead to torsion. Rarely, other tumors such as mesoappendiceal lipoma,46 decidual change,47 and a short mesoappendix48 have been associated with torsion. Volvulus of the appendix is inevitably less common. Again in adults it is usually secondary although, like torsion, in infants the cause is less clear.49, 50
Intussusception
Although rare, this tends to have a male predominance with a peak around puberty.51 Clinically it is usually an incidental finding at appendectomy, although a coiled spring appearance on barium enema is occasionally found.52 Like torsion, there is invariably an underlying cause for the intussusception. In young males it is usually lymphoid hyperplasia, and this may be unassociated with underlying acute inflammation. Other causes include epithelial tumors both benign and malignant, carcinoids, endometriosis, foreign bodies including fecaliths, diverticula hemorrhage into the wall, and sometimes even otherwise uncomplicated acute appendicitis.53, 54, 55, 56, 57, 58, 59 Ischemia may be present at the apex but transmural necrosis is rare.60 Sometimes they may be visualized colonoscopically and mistaken for polyps.61
Surprisingly, in 1941 McSwain described four variants of appendiceal intussusception, the first being invagination of the tip into the proximal lumen of the appendix, the second being invagination of the base or the midpart of the appendix into or toward the cecum, and the third being a reverse invagination so that the distal appendix is circumferentially everted and rolled over the proximal external surface, rather like pulling a sock over ones foot. Finally the entire appendix may be inverted into the cecum, and this may also be accompanied by an ileocecal intussusception, the inverted appendix and ileocecal valve then forming the apex of the intussusception.62 For the pathologist, the major concern is to identify the cause of the intussusception.
Intussusception may also rarely occur in the inverted appendiceal stump; this is discussed in the section dealing with complications following appendectomy.
IATROGENIC USE OF THE APPENDIX
Appendicovesicostomy (Mitrofanoff procedure): This is an operation in patients, usually children, with neurogenic bladder, the epispadias-extrophy complex, or a cloacal anomaly, that allows catheterization of the bladder through an otherwise continent stoma formed from the appendix and small cuff of cecum, the other end of which has been reimplanted into the bladder. The appendix is separated from its attachment to the cecum, while maintaining its blood supply, and an opening is created at its blind end. One end is anastomosed to the urinary bladder, and the other to the skin to form a stoma. It has also been performed in adults with urethral carcinoma and in paraplegic patients. Other variations on the theme exist including using cecum or terminal ileum and even a Meckel’s diverticulum to allow catheterization.
Appendicostomy (Malone procedure) is used in patients with fecal incontinence that requires regular enemas. It consists of connecting the appendix to the umbilicus, and creating a valve mechanism that allows catheterization of the appendix for the enema fluid, but avoids leakage of stool through it. It allows independence, especially to children wanting independence, to manage their own problems. Complications include adhesions, but acute appendicitis63 and inflammatory polyps with granulation tissue can also occur.64
ACUTE APPENDICITIS
Acute appendicitis is a disease that affects primarily children and young adults of Westernized countries. The peak age is between 10 and 25 years, but the tails of the curve are extensive in both directions. Although the overall mortality is very low (< 1%), it rises significantly in the very young and the very old, probably as a result of delay in diagnosis. Neonatal appendicitis is rare and presents atypically, such as with sepsis or ascites and has a high mortality. If laparotomy is carried out it may be discovered but is rarely diagnosed preoperatively. It is usually associated with prematurity or comorbidities, primarily inguinal hernias, possibly raising the question of whether ischemia may be involved.65 There is a male preponderance, with a male to female ratio of about 1.4:1, and the overall lifetime risk is thought to be in the order of 8.6% for males and 6.7% for females in the United States,66 although the incidence may be slowly falling. Previously women were much more likely to have a normal appendix removed because of pain from associated gynecologic abnormalities, particularly in the immediate postpubertal decade, when up to half of the appendices removed in women may be normal.67 However, the use of imaging techniques has markedly reduced the number of incorrect diagnoses.
Clinical Features
Typically the patients present with central abdominal pain that moves to the right lower quadrant. Clinically perforation is suspected when the pain becomes diffuse, with guarding and rigidity. In the elderly the mortality may exceed 15%; other contributing factors include cardiovascular disease associated with high perioperative mortality.68 When it occurs in the first month of life, a mass is invariably present and the mortality is well over 50%.13 Appendicitis in the neonatal period is invariably associated with peritonitis; however, under these circumstances it may not be simply acute appendicitis, but may present as a complication of either necrotizing enterocolitis or Hirschsprung’s disease.69, 70, 71 Almost all of the morbidity and mortality from acute appendicitis come from its complications, which result from perforation. Following perforation there may be diffuse peritonitis or local abscess formation and sometimes septicemia. Subsequent adhesions may cause obstruction. Rarely, the inverted appendiceal stump may be mistaken for a polyp and resected.
Pathogenesis. Appendicitis seems to be a disease primarily of the 20th century, the first documented appendectomy being carried out only in 1580. The diagnosis became fashionable at the beginning of the 20th century following the delay in the coronation of King Edward VII in England in 1902 because of his appendiceal abscess.72 The prevalence of appendicitis increased until the 1940s and seems to have very slowly declined since then, although still a common disease.
The two major predisposing factors in appendicitis are obstruction, often due to a fecalith, and infection. Numerous organisms have been implicated in the etiology. Fusobacterium species appears most strongly implicated as discussed subsequently. Reactivation of Cytomegalovirus (CMV) has also been found, although its role is unclear.73
The role of diet has also been called into question, especially regarding an association with fecaliths. Western low-bulk diet has also been implicated,74 but controlled studies show no significant differences between those developing appendicitis and matched controls.75 One Swedish study showing an apparently
decreasing rate of appendicitis was associated with a reduction in dietary fiber.76
decreasing rate of appendicitis was associated with a reduction in dietary fiber.76
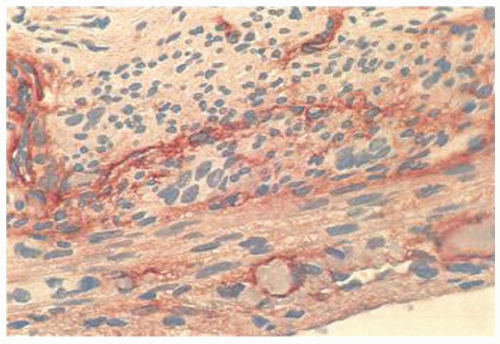
Other rare causes of appendicitis include vasculitis, which can be associated with rheumatoid disease, lupus, polyarteritis, and Henoch-Schonlein purpura.77, 78, 79, 80 Also enterocolitic lymphocytic phlebitis can affect the appendix. One patient has been described also having a lymphocytic appendicitis and enteritis81 (Fig. 15-11).
Obstruction It is not surprising that obstructing the lumen of the appendix results in distal inflammation, often with an ischemic component, that can proceed to perforation. Further, if there was no luminal obstruction then pus could drain freely into the cecum and not result in the complications that otherwise occur. Experimental exteriorization of the appendix, primarily in animals, but occasionally in patients after resection for colonic carcinoma, is often followed by occlusion of the appendix base yet without interference with the vascular supply. This results in acute necrotizing inflammation, supporting the notion that appendiceal obstruction plays a major role in the pathogenesis of acute appendicitis.82, 83, 84
Appendiceal obstruction, if present, is usually intraluminal, resulting from a fecalith; however, other causes of intraluminal obstruction, such as orange pips, cherry pits, or other food fragments which may become laminated and calcified, foreign objects such as bullets, bezoars, intrauterine contraceptive devices, barium from barium studies, and possibly parasites may all cause luminal obstruction.85, 86, 87, 88 Obstruction may also result from swelling of the wall of the appendix. In children in the second decade of life, it may be the result of lymphoid hyperplasia, which is maximal during this period,89, 90 (Fig. 15-3) but tumors of all types are often the presenting feature of appendiceal neoplasms, including even Kaposi sarcoma in patients with acquired immunodeficiency syndrome (AIDS).91
Rarely, extra-appendiceal obstruction may result from torsion, volvulus, or fibrous bands and adhesions affecting the appendix from prior intra-abdominal surgery. Extra-appendiceal tumors—for instance, cecal carcinoma, endometriosis, or foreign bodies—may all incidentally involve the appendix and occasionally appear to precipitate acute appendicitis.
Rarely both primary and secondary tumors, including endometriosis, and other rare tumors may cause obstruction however, the role of fecaliths in the genesis of appendicitis has changed over the last 50 years. Studies from the late 1930s suggest that a fecalith may have been present in two-thirds of patients with appendicitis.92 During the intervening decades, this figure has gradually dropped by about 10% per decade and is now probably on the order of 20% to 30%. In one study it was as low as 6%.93
The issue of what happens to appendices with fecaliths before they become inflamed is interesting. Presumably they are either expelled, stay put, or result in acute appendicitis. One study carried out laparoscopic appendectomy of normal-looking appendices in the absence of any other obvious pathology. Thirteen percent of histologically normal appendices contained fecaliths, and thirty percent of appendices with fecaliths were histologically normal. Surprisingly (or not), this appeared to be an effective treatment for recurrent symptoms in those cases with a fecalith. Only one (5%) of those with a fecalith had recurrent pain postoperatively compared with 50% of those without a fecalith but with a histologically normal appendix. This suggested that some patients really did develop appendicular colic from their fecaliths, and that this could be prevented by appendectomy. Pain was either right iliac fossa pain or central abdominal
pain shifting to the right iliac fossa in both those with acute appendicitis and those with only a fecalith.94
pain shifting to the right iliac fossa in both those with acute appendicitis and those with only a fecalith.94
Infection There is little doubt about the role of infection as a perpetuating force in acute appendicitis once the mucosa is ulcerated. But the question of whether they initiate typical acute appendicitis is a major issue. Most pathologists are well aware of the similarity of early acute appendicitis and acute infectious colitis, and many would even have done an occasional special stain looking for them. Yet proving it is far from easy. The best candidate appears to be Fusobacterium species which has been found specifically using rRNA-based fluorescence in situ hybridization. Fusobacteria (mainly Fusobacterium nucleatum and Fusobacterium necrophorum) were found in epithelial cells and submucosal infiltrates in 62% of patients with proven appendicitis.95 The presence of the organism correlated with the severity of the appendicitis. After the initial paper, appendices from several countries were examined with similar results, albeit better in Carnoy’s than formalin-fixed appendices.96 Having identified what appears to be at least one possible major infectious cause of acute appendicitis, it would not be surprising to find others. In another study, evidence of CMV infection/reactivation was also found in about two-third of patients with acute appendicitis, although its role is unclear.73
In patients with luminal obstruction (fecalith or other causes) the mucosal ischemia would allow invasion of normal appendiceal flora. The surprise perhaps is that these were never isolated from peritoneal cultures. However culture of appendiceal abscesses or peritoneal fluids invariably reveals fecal-type organisms, which in most cases are mixed, although occasionally apparently pure cultures, particularly of Escherichia coli, are found. However, other organisms, in particular anaerobic organisms, are frequently cocultured. Occasionally other enteric infections may be associated with acute appendicitis, such as those caused by Salmonella, Shigella, or Campylobacter.97, 98, 99, 100, 101 However, all of these organisms most likely cause a pure mucosal appendicitis as they do elsewhere in the gut, and transmural inflammation from them is relatively rare. Nevertheless, many of these organisms such as Salmonella have long been implicated as an occasional cause of appendicitis.
There is a similar possibility that lymphoid hyperplasia may be related to underlying viral infections such as adenovirus, measles, and other enteric viruses. However, attempts to isolate viruses from hyperplastic appendiceal lymphoid tissue at appendectomy have been largely unsuccessful. Nevertheless when appendicitis is present in the absence of luminal obstruction, virus-like inclusions may occasionally be seen in the epithelium, particularly in children; culture of material from these appendices may also yield viruses such as adenovirus.102, 103
Some forms of acute appendicitis are sufficiently characteristic to be considered separately, including those related to Yersinia infection. In addition, acute appendiceal diverticulitis may proceed rapidly to perforation (see previous discussion).
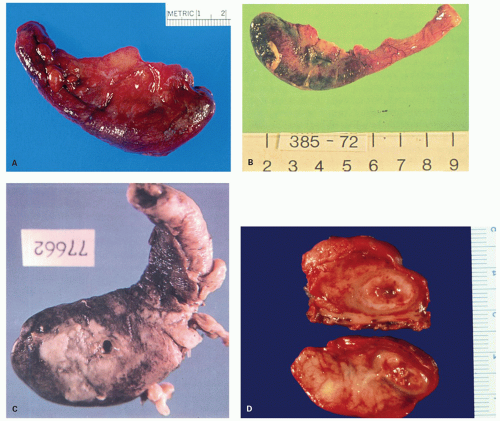
Gross Appearance
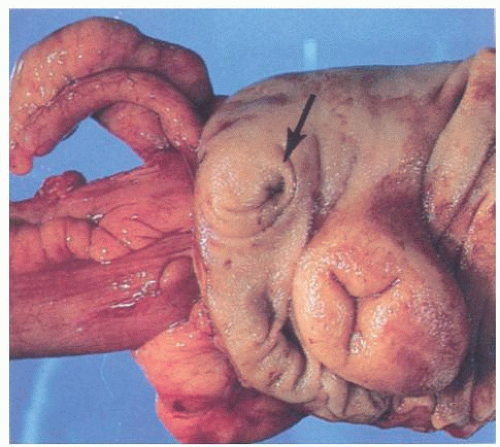
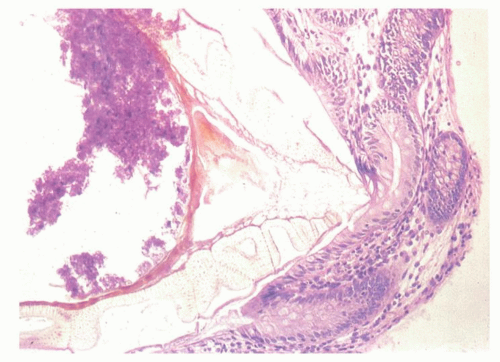
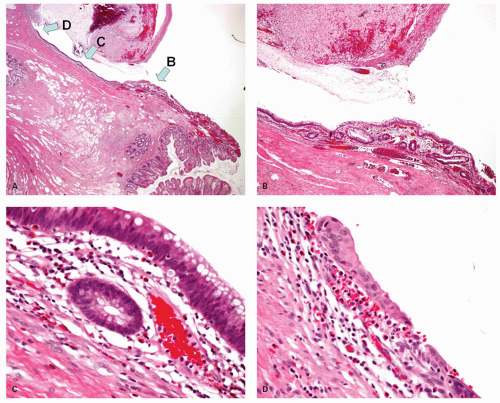
The macroscopic appearance of the appendix in acute appendicitis varies with the stage of the inflammation. When inflammation is confined to the mucosa, and sometimes to the submucosa, the appendix may look macroscopically almost normal, although the vessels on its serosal surface and in the mesentery may appear a little congested producing an injected appearance. With progression of the acute inflammatory process into and through the muscularis propria, the appendix initially takes on a red hue, which becomes bluish/dark purple and ultimately plum colored (Fig. 15-12A,B). The serosa may lose its sheen and become granular; sometimes it is covered with a cream-yellow fibrinopurulent exudate. Lymph nodes may be enlarged and congested but are usually not biopsied. Ultimately the appendix may become black and gangrenous. A perforation in the appendix may also be present, often as a well-circumscribed, punched-out hole (Fig. 15-12C). If a fecalith is present, the appendix may be almost entirely normal proximally, but in its distal part the lumen is dilated and filled with pus or liquid fecal material, and sometimes is hemorrhagic. If the gross appearances suggest relatively focal involvement in one part of the appendix, a possibility of it being caused by an underlying fecalith or a diverticulum should be borne in mind and the lesion sought. In some patients the tip of the appendix may be very thin and atrophic, suggesting that prior episodes of appendicitis may have occurred. The inflammation may extend into the mesoappendix for a varying distance and may be overtly purulent (Fig. 15-12D). The appearance of the mucosal surface and the wall of the appendix usually becomes evident when a longitudinal incision is made in the distal 2 cm of the appendix. Serial slicing of the more proximal appendix at 2-mm intervals may reveal not only a fecalith but also other causes of obstruction including polyps, foreign bodies, diverticula and, rarely, tumors. Occasionally thrombosis may be observed in the vessels on the surface of the appendix or mesoappendix; in rare cases, it is yellowish and purulent.
Histology
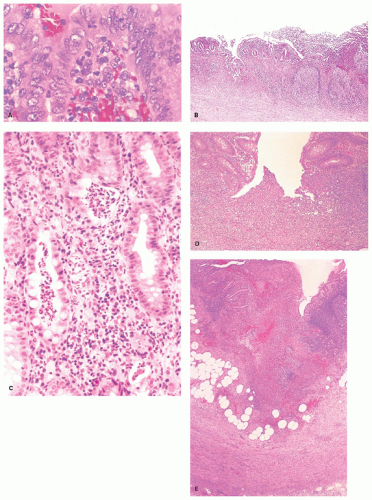
Acute appendicitis begins within the mucosa. The earliest change seems to be margination of mucosal capillary channels with neutrophils which migrate into
the lamina propria. These migrate beneath and into the surface epithelium (Fig. 15-13A), causing initially an erosion (Fig. 15-13B); into adjacent crypts, forming crypt abscesses (Fig. 15-13C) and ulcers that resemble aphthoid ulcers (Fig. 15-13D); and into lymphoid follicles, causing deep suppuration (Fig. 15-13E). This process proceeds rapidly to full-thickness mucosal ulceration, and crypt abscesses may focally burst downward into the submucosa and then into the muscularis propria. If this process occurs within a diverticulum, perforation into the submucosa may be tantamount to free perforation. Myocytolysis of the muscle and acute inflammation of the subserosa, visceral peritoneum, and mesoappendix may follow. In extreme forms, the entire appendix may look like a bag of pus and transmural necrosis may ensue. Rarely, suppuration may continue into the appendiceal veins leading to pyelophlebitis. Sometimes the number of eosinophils can be very prominent in the infiltrate, which could be a manifestation of an eosinophilic enteritis or a parasitic infection.104, 105, 106, 107 Mucosal ulceration is evident in all cases of acute appendicitis unless a periappendicitis resulted from the spread of inflammation from an adjacent organ, or interval appendectomy has been carried out, when the ulcers may have resolved. Inflammation often involves the serosa (serositis or peritonitis) with inflammatory exudate, fibroblastic and capillary proliferations, and mesothelial reaction. The mesothelial reaction can be fairly marked in some cases and mimic giant cells
and granulomas or epithelial malignancy, especially when keratin stains are performed. The stromal cells can appear pleomorphic and can also lead the unwary to a diagnosis of malignancy. Occasionally multinucleated giant cells may be seen in the submucosa or subserosa that mimic virally infected cells, but are reacting stromal, endothelial, or mesothelial cells (Fig. 15-13F,G). The latter can be treacherous as keratin staining may create a false impression of metastatic carcinoma, especially from a gastric or breast primary that are notorious for infiltrating single cells, and especially if the patient really has a history of one of these diseases. Other mesothelial markers help resolve the situation (Fig. 15-13G). Submucosal lymphatics may be plugged in a manner, which, at lower power, mimics a carcinoid (Fig. 15-13H).
the lamina propria. These migrate beneath and into the surface epithelium (Fig. 15-13A), causing initially an erosion (Fig. 15-13B); into adjacent crypts, forming crypt abscesses (Fig. 15-13C) and ulcers that resemble aphthoid ulcers (Fig. 15-13D); and into lymphoid follicles, causing deep suppuration (Fig. 15-13E). This process proceeds rapidly to full-thickness mucosal ulceration, and crypt abscesses may focally burst downward into the submucosa and then into the muscularis propria. If this process occurs within a diverticulum, perforation into the submucosa may be tantamount to free perforation. Myocytolysis of the muscle and acute inflammation of the subserosa, visceral peritoneum, and mesoappendix may follow. In extreme forms, the entire appendix may look like a bag of pus and transmural necrosis may ensue. Rarely, suppuration may continue into the appendiceal veins leading to pyelophlebitis. Sometimes the number of eosinophils can be very prominent in the infiltrate, which could be a manifestation of an eosinophilic enteritis or a parasitic infection.104, 105, 106, 107 Mucosal ulceration is evident in all cases of acute appendicitis unless a periappendicitis resulted from the spread of inflammation from an adjacent organ, or interval appendectomy has been carried out, when the ulcers may have resolved. Inflammation often involves the serosa (serositis or peritonitis) with inflammatory exudate, fibroblastic and capillary proliferations, and mesothelial reaction. The mesothelial reaction can be fairly marked in some cases and mimic giant cells
and granulomas or epithelial malignancy, especially when keratin stains are performed. The stromal cells can appear pleomorphic and can also lead the unwary to a diagnosis of malignancy. Occasionally multinucleated giant cells may be seen in the submucosa or subserosa that mimic virally infected cells, but are reacting stromal, endothelial, or mesothelial cells (Fig. 15-13F,G). The latter can be treacherous as keratin staining may create a false impression of metastatic carcinoma, especially from a gastric or breast primary that are notorious for infiltrating single cells, and especially if the patient really has a history of one of these diseases. Other mesothelial markers help resolve the situation (Fig. 15-13G). Submucosal lymphatics may be plugged in a manner, which, at lower power, mimics a carcinoid (Fig. 15-13H).
If appendicitis is treated conservatively, subsequent appendectomy will reveal a variety of microscopic changes, depending on the stage of resolution. The appendiceal mucosa may have been completely ulcerated or obliterated, or may show regenerative changes; there is often a marked inflammatory and fibroblastic reaction in the submucosa. Residual hemorrhage is usually present in all areas of the wall, and numerous hemosiderin-laden macrophages may be present, particularly in the submucosa and immediately outside the muscularis propria, where this can still be identified. However it may be largely replaced by fibrous tissue, either in its entirety or focally. Occasionally reepithelialization occurs around abscess cavities in the submucosa. If there is no residual acute inflammation, this is probably best called resolving appendicitis rather than chronic appendicitis. Although the latter term is accurate, it is so ambiguous that it requires definition whenever it is used and is therefore of limited value. We try and resist using the term “non-specific” before “inflammation”, as unless a cause can be identified in the section, usually infective, all inflammation is non-specific, so it is a historical substitute for “don’t ask us as because we don’t know”.
Resolution of Acute Appendicitis
Crypt architectural distortion. This is common in appendectomy specimens and suggests at least a previous episode of mucosal ulceration with an architectural
abnormality which focally may resemble that seen in ulcerative colitis. Usually there is underlying dense submucosal fibrosis and a duplicated muscularis mucosae.
abnormality which focally may resemble that seen in ulcerative colitis. Usually there is underlying dense submucosal fibrosis and a duplicated muscularis mucosae.
Localized epithelial hyperplasia (reactive hyperplasia). This is a thickened mucosa without dysplasia or serrations and is commonly, seen in appendectomies for acute appendicitis, in which the luminal epithelium is thickened, often visible grossly. It often occupy much of the appendiceal lumen although sometimes they are also quite localized, but it is unclear if it can cause sufficient obstruction and therefore be involved etiologically in acute appendicitis. If it is a reactive lesion following acute appendicitis, it appears unlikely that it would be present prior to the clinical episode— unless the appendicitis was recurrent so that the opportunity was present.
Submucosal fibrosis. It should be recalled that in neonates and children, the submucosa is completely fibrous and adheres directly to the muscularis mucosae on one side and the muscularis propria on the other, so that in children the term submucosal fibrosis is probably best avoided. With increasing age partial fatty replacement of the submucosa occurs. Dense submucosal fibrosis in adults, which is particularly focal may indicate a prior episode of appendicitis; occasional bifid crypts may also be present to add weight to that interpretation. In some patients, an iron stain may reveal hemosiderin-laden macrophages, which again indicates previous inflammation.
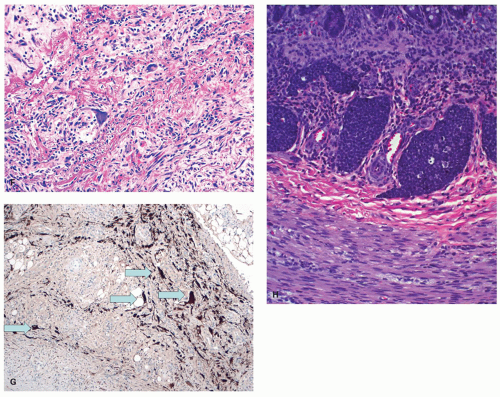
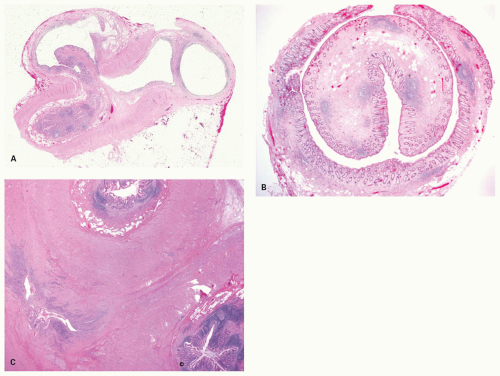
Luminal obliteration (obliterative fibrosis) and neuroma of the appendix. Although obliteration of the appendix is well recognized as a long-term sequel of acute appendicitis that has resolved, about one-quarter of these cases will also have neuronal hyperplasia. This has variously been called neuroma, neurogenic appendicopathy, neurofibroma, and neurogenic appendicitis.108, 109, 110 The most common type of proliferation occurs when there is central obliteration of the lumen with a relatively normal-sized appendix (Fig. 15-14). However, the neural proliferation may also be nodular. The least common variant is one in which the proliferation is confined to the mucosa of the appendix. Masson110 originally suggested that this lesion may be a precursor to appendiceal carcinoid, as it invariably contains small clusters of enterochromaffin cells which immunostain for serotonin and other neuronal markers.111 Sometimes these cells occur in small nests, the inference being that if these nests grow large enough, they will form a carcinoid tumor. This pathogenetic mechanism is said to explain the fibrous obliteration that is seen in some appendiceal carcinoids; the cutoff seems to be rather arbitrary.
One study observed neurogenous hyperplasia in almost 90% of resected appendices and suggested a continuum from appendices with an intact lumen, but with intramucosal neurogenous hyperplasia which coexisted with submucosal and muscular growth, up to obliterated appendices with tangles of nerves and fibrous tissue.108
Terminology in Acute Appendicitis
There are numerous names applied to acute inflammatory pathology in the appendix; however, the choice of terminology has little significance when features of typical appendicitis with transmural acute inflammation are identified and the cause of abdominal pain is explained.
At one extreme, when occasional neutrophils are present in the lumen and multiple sections fail to reveal evidence of mucosal inflammation, we usually choose not to report these as acute appendicitis, although we mention the presence of luminal neutrophils and submit the remainder of the appendix to exclude typical acute appendicitis elsewhere, and also ensure that extrinsic inflammation affecting
the appendix as serosal inflammation is not present. When there is inflammation of the mucosa, but with either only occasional neutrophils in the lamina propria or epithelium, or only the mucosa has become purulent, the term acute mucosal appendicitis is sometimes used. Where the inflammation is transmural with numerous neutrophils simulating abscess formation the term acute suppurative appendicitis has been used, although some prefer acute phlegmonous appendicitis. To these terms are usually added with serosal inflammation or with perforation when appropriate. In an appendix that is virtually black or very dark plum colored, or frankly necrotic, the term gangrenous appendix or gangrenous appendicitis can be used. The presence of serositis is worth documenting given its correlation with right lower quadrant pain, and therefore affirmation that the appendix was likely the cause of clinical symptoms present. The term “subacute” has mercifully been expunged from the lexicon. Some used it when an excess of eosinophils was present, but most, and especially clinicians, had no idea what this term meant histologically.
the appendix as serosal inflammation is not present. When there is inflammation of the mucosa, but with either only occasional neutrophils in the lamina propria or epithelium, or only the mucosa has become purulent, the term acute mucosal appendicitis is sometimes used. Where the inflammation is transmural with numerous neutrophils simulating abscess formation the term acute suppurative appendicitis has been used, although some prefer acute phlegmonous appendicitis. To these terms are usually added with serosal inflammation or with perforation when appropriate. In an appendix that is virtually black or very dark plum colored, or frankly necrotic, the term gangrenous appendix or gangrenous appendicitis can be used. The presence of serositis is worth documenting given its correlation with right lower quadrant pain, and therefore affirmation that the appendix was likely the cause of clinical symptoms present. The term “subacute” has mercifully been expunged from the lexicon. Some used it when an excess of eosinophils was present, but most, and especially clinicians, had no idea what this term meant histologically.
We usually do not use the term chronic appendicitis because of its ambiguity, so if used demands a description and interpretation. The appendix normally has a considerable amount of chronic inflammatory tissue in the lamina propria, both as mucosal lymphoid nodules and lamina propria plasma cells. This term may legitimately be used with specific chronic infections or involvement by inflammatory bowel disease (IBD) or as a synonym for resolving appendicitis; we prefer the latter term. In adults we occasionally use the term submucosal fibrosis where that is present, but not in children because this is a normal finding. Similarly although we accept the presence of neural hyperplasia, we do not always stain obliterated appendices to demonstrate its presence and tend to utilize the simple descriptive term obliterated appendix (partial if that is the case).
In cases where there is definite periappendicitis in the absence of other features of acute appendicitis, the clinician should be warned that appendix may have been secondarily involved due to pathology in other adjacent organs and abdominal pain may persist. One should also be careful not to use the term periappendicitis for the peritoneal neutrophil infiltrate induced by surgical manipulations, but reserve it for severe inflammatory cases where it is reasonable to attribute the patient’s right iliac fossa pain to this inflammation. Under these circumstances other diseases, such as gynecologic inflammation or rarely Crohn’s disease, may have to be considered.
Unusual variants of appendicitis
Appendiceal Diverticulitis Inflammation into the submucosa in a diverticulum is tantamount to perforated appendix because of the lack of a muscularis propria in most appendiceal diverticula. A focal perforation with relatively localized inflammation should therefore prompt a careful search for other evidence of diverticulitis, as in about half the cases the diverticula are multiple (see previous discussion). Even if not identified, a very focal fistula-like appearance is likely very good grounds for regarding this as an ulcerated diverticulum that has perforated.
Perforation with a Foreign Object Rarely, acute appendicitis is the result of impaction of sharp objects such as fish or chicken bones in the wall of the appendix, causing acute appendicitis and perforation. Alternatively a seed, pith, or other food item may be present in the lumen, causing obstruction with subsequent distal inflammation (Fig. 15-15). Fistulae can occur from extrinsic involvement by other diseases and foreign objects including surgical sponges (gossypiboma).
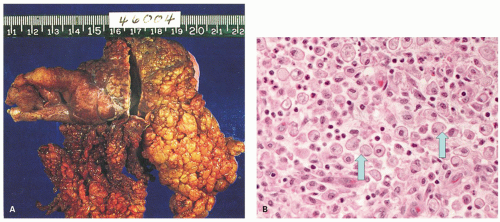
Agranulocytic (Neutropenic) Appendicitis Rare cases of appendicitis occur in neutropenic patients, especially children with mucosal necrosis and hemorrhage but without an accompanying acute inflammatory reaction (Fig. 15-16). This usually takes place on a background of severe neutropenia as a result of chemotherapy for underlying malignant disease, but sometimes occurs when the neutrophil count has recovered. It is unclear if epithelial loss is partly the result of chemotherapy or due to the inability to mount an acute inflammatory response. The situation is similar to neutropenic colitis. It is clear that these patients can be managed conservatively unless there is evidence of perforation, and also that they can undergo an appendectomy without a major risk. The intensity of the neutropenia may also play a role as may the underlying disease and the general well-being of the patient.112, 113, 114, 115, 116
Intussusception Rarely, the appendix may be the apex of an intussusception into the large bowel. This is discussed previously.
Inflammation in Incidental Appendectomies Incidental appendectomy is frequently carried out on a routine basis during surgery for various causes such as cholecystectomy or ovarian cysts or neoplasms. In most of these cases there is no abnormality, but occasionally there are surprises. Sometimes luminal pus is present, and careful section of these appendices will frequently reveal either an erosion or a focus of mucosal ulceration. We tend to report these descriptively, for example, as “acute appendicitis with focal mucosal erosion.” Although this process may presumably go on to full clinical acute appendicitis, particularly in the presence of a fecalith, such changes are probably relatively frequent and are probably more likely to resolve spontaneously than to progress.
Appendicitis in Appendix Stumps Because many appendices are removed laparoscopically and are simply transected and not inverted, the possibility of appendicitis of the residual appendix stump always remains. It is particularly likely to happen if the appendix is not transected close to its base. A history of prior appendectomy clearly results in delay in the diagnosis as the possibility may not even be considered. A diagnosis can be made with an ultrasound or computed tomography scan, and if diagnosed early, laparoscopic or open completion appendectomy can be performed. If diagnosis is delayed, perforation can occur requiring a more extensive resection.117, 118, 119 Conversely inverted appendiceal stumps removed by conventional (open) appendectomy may be misdiagnosed as adenomas or other polyps and removed, which may result in perforation (see Fig. 15-17). Most colonoscopists are well aware of identifying the appendiceal orifice and being very suspicious of any “polyp” in the vicinity of where the orifice should be.120 Issues associated with the appendiceal stump are discussed in more detail subsequently.
A related clinical trap is in not suspecting appendicitis due to a second appendix in a patient who has undergone previous appendectomy. This is discussed previously.
Issues with Appendicitis and a Practical Approach
The pathologist has three major roles in acute appendicitis. The first is to determine if there is appendiceal pathology, the second its type and finally any implications for future patient management.
Most cases of acute appendicitis present no real problem because there is a suppurative process involving the mucosa, the submucosa, and usually the entire
thickness of the appendiceal wall. However when the inflammation is only mucosal and submucosal, it is more difficult to correlate this with right iliac fossa pain unless a fecalith is also present (see previous discussion); nevertheless, if suppuration is limited to the mucosa, we are reluctant to make an association other than with periumbilical pain, implying that there may be an alternative cause for the patient’s symptoms. However the rest of the appendix should be blocked and examined to ensure that focal acute appendicitis, such as might occur in a diverticulum, is not present.
thickness of the appendiceal wall. However when the inflammation is only mucosal and submucosal, it is more difficult to correlate this with right iliac fossa pain unless a fecalith is also present (see previous discussion); nevertheless, if suppuration is limited to the mucosa, we are reluctant to make an association other than with periumbilical pain, implying that there may be an alternative cause for the patient’s symptoms. However the rest of the appendix should be blocked and examined to ensure that focal acute appendicitis, such as might occur in a diverticulum, is not present.
It is not unusual for a layer or two of neutrophils to be present in the subserosa as a result of surgical manipulation, although this is now less common with laparoscopic removal. Such minimal acute periappendicitis should not be interpreted as the cause of the patient’s pain or as evidence of serosal involvement by extension from the lumen.
Occasionally appendices contain only a little luminal pus, sometimes in association with other objects such as worms. Again, however, in the absence of inflammation through the mucosa, we are reluctant to attribute clinical symptoms to such mild pathology.
This issue is not infrequently in practice, where an appendectomy has been performed for abdominal pain, but histologically the appendix appears near normal or the inflammatory changes are minimal. The questions arising is whether (a) a few neutrophils seen in the interstitium of the submucosa and/or muscularis propria are enough to explain the abdominal pain? (b) Does vascular congestion and margination of neutrophils mean anything? (c) What if only the mucosa is inflamed?
While a few papers have been written on the issue, no specific guidelines have been proposed nor are there good studies that can answer these questions. Standard texts suggest that neutrophils are absent in normal appendices, especially in submucosa and muscularis; however, good studies are few. One study showed that neutrophils are indeed absent in the muscularis propria and very rare (<1/high power field [HPF]) in the submucosa of normal appendices.121 In this study, neutrophils were increased in the mucosa (mean 12.27/HPF) in cases of early appendicitis compared to the normal appendices (mean 5.46/HPF)121 (this was regarded as a high figure). Increased margination of neutrophils was also seen in the subserosal postcapillary venules (PCV) in cases of early appendicitis (mean 9.47/10 PCV) compared to normal appendices (mean 6.72/10 PCV). In early appendicitis neutrophils were not seen in the muscularis propria or submucosa, thus the presence of neutrophils in these layers outside of vessels is a strong indicator of appendicitis.121 On the other hand when typical changes of acute appendicitis are lacking, other causes for abdominal pain can be found, if carefully looked for.122
The best practical approach whenever such a situation arises is to first ensure that the entire appendix has been submitted for histology and sign out the cases as “appendix with minimal inflammatory changes or near-normal histology” with an explanatory note which should urge the clinician to keep looking for alternate sources of pain in the abdomen. If the pain in the abdomen resolves following appendectomy and nothing else is found, only then can one safely assume that the appendix was the likely source despite minimal changes. Over the years, we have seen cases where the inflammation in the appendix was minimal and symptoms did resolve following appendectomy.
In carrying out audits, appendectomies without any real pathology should probably not exceed about 20%.123 The highest rate of normal appendectomies occurs in women of childbearing age. In one study, almost 50% of the appendices removed in women in this age group were normal, a figure that rose to 59% in women between 15 and 25 years of age. However, appendiceal imaging has markedly increased the accuracy of appendiceal versus gynecologic pathology.124
Complications of Acute Appendicitis
Perforation
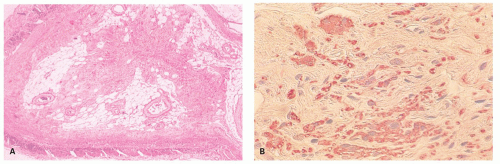
This is a relatively common complication of appendicitis, occurring in about 20% to 30% of patients overall, although it is much higher in the young and the old. Several studies suggest that an important predisposing factor for perforation is delay in seeking medical attention, which may be of the order of one to two additional days. Delay by the physician in carrying out appendectomy is probably only a small factor. Perforation can be diagnosed preoperatively because of the signs, symptoms, and laboratory values, and consists of either free perforation or localized abscess. Culture usually reveals mixed aerobic and anaerobic organisms, usually including E. coli and Bacteroides, but other fecal organisms may also be present. In about a quarter of these cases, a single organism is found. The appearances of perforation were described earlier. Complications of perforation include abscess formation, particularly in the pelvis, pericolic gutters, and subhepatic and subphrenic spaces. Perforation in the absence of marked mucosal disease raises the question of a perforated diverticulum.
Appendiceal epithelium growing along the external surface of the appendix. Some appendices are found to have not just a perforation, but apparently normal mucosa growing along the external surface of the appendix. This can vary from a thin layer of attenuated epithelium to a full mucosa with lamina propria (Fig. 15-18). Clearly for this to occur, time is required presumably in days or weeks, for the mucosa
to grow and regenerate following perforation. So this is a complication seen primarily in interval appendectomies or in those whose appendectomy is delayed. It is more likely to happen in retrocecal appendices where perforation is less likely to result in peritonitis due to local containment.
to grow and regenerate following perforation. So this is a complication seen primarily in interval appendectomies or in those whose appendectomy is delayed. It is more likely to happen in retrocecal appendices where perforation is less likely to result in peritonitis due to local containment.
The issue is always to ensure that the lining epithelium is non-neoplastic. When columnar this is usually not a problem, but if attenuated it is necessary to trace the mucosa back to where it originated from and ensure that this is also nondysplastic. Should the mucosa be dysplastic, this raises the question of whether the patient is at risk of pseudomyxoma. However in the perforations that we have seen, it has not been an issue and is not accompanied by mucin extravasation, except in one instance of what was clearly a ruptured benign mucocele. However reactive hyperplastic epithelium can be a problem as the dysplasia is subtle, but this epithelium is usually quite thickened as opposed to mucinous neoplasms in which the epithelium is usually no thicker than normal appendiceal mucosa.
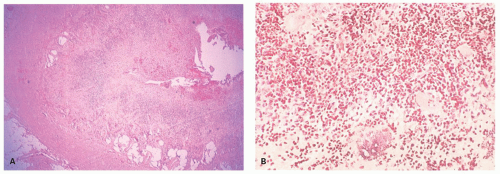
Periappendiceal abscesses and productive periappendiceal fibrosis. These are relatively uncommon and occur in <5% of patients with acute appendicitis, particularly those who have delayed in seeking medical help. They are seen relatively infrequently by pathologists because there is a tendency to treat
these lesions by simple drainage and usually interval appendectomy several weeks later, unless it is technically simple to do it at the time of the initial laparotomy. However increasingly complicated appendiceal disease is treated with laparoscopic surgery or open laparoscopy, but the number of complex appendectomies seems to be changing a little. Nevertheless appendiceal abscesses are associated with a relatively high morbidity, and up to a third of patients will develop complications such as wound infection, fecal fistula, recurrent abscess formation, and localized small bowel obstruction soon after drainage; however, a similar series of complications can also be expected following interval appendectomy. While these figures make treatment difficult, the failure to carry out interval appendectomy is also associated with a high incidence of recurrent abscesses and sometimes recurrent appendicitis. Periappendiceal abscesses seem therefore associated with a high rate of complications irrespective of how they are managed.
these lesions by simple drainage and usually interval appendectomy several weeks later, unless it is technically simple to do it at the time of the initial laparotomy. However increasingly complicated appendiceal disease is treated with laparoscopic surgery or open laparoscopy, but the number of complex appendectomies seems to be changing a little. Nevertheless appendiceal abscesses are associated with a relatively high morbidity, and up to a third of patients will develop complications such as wound infection, fecal fistula, recurrent abscess formation, and localized small bowel obstruction soon after drainage; however, a similar series of complications can also be expected following interval appendectomy. While these figures make treatment difficult, the failure to carry out interval appendectomy is also associated with a high incidence of recurrent abscesses and sometimes recurrent appendicitis. Periappendiceal abscesses seem therefore associated with a high rate of complications irrespective of how they are managed.
Productive periappendiceal fibrosis is known by a variety of synonyms, including chronic tumefactive ligneous perityphlitis, appendicular granuloma, ligneous cecitis, and pseudoneoplastic appendicitis, all of which imply the presence of a large, hard (ligneous, woody) fibroblastic inflammatory mass which may mimic a neoplasm and adhere to surrounding structures. Microscopically apart from proliferative fibroblastic tissue and granulation tissue, there are frequently numerous foreign body giant cells and granulomas, presumably as a reaction to appendiceal contents which have become organized. Sometimes it is impossible to identify the appendix in these masses. Under these circumstances, it is often easiest to identify the appendiceal orifice within the cecum, gently insert a probe, and open up the appendix longitudinally as far as it will go. Sometimes the appendix simply disappears into this mass within a centimeter or so. Occasionally such masses contain fecaliths. Although they are disconcerting for the surgeon, there is little to suggest that these are anything other than organizing inflammatory appendiceal abscesses. Disconcerting for the pathologist is that there may be numerous lymphoid aggregates similar to those seen around complex diverticular disease that can mimic Crohn’s disease, and fistulae may also be present. However if the patient has no known history of Crohn’s disease, there is no justification for making that diagnosis.
Formation of multiple lumina (lumens). While these may represent duplications, more frequently multiple lumina, or apparent multiple lumina, are found following acute appendicitis when abscesses reepithelialize producing sacculations, septa formation, or invaginations of the lumen or serosal fibrosis results in the appendix being folded back on itself or another organ (Fig. 15-19).
Escaped fecalith. This is an unusual complication in which patients who have had a perforated appendix removed present again several weeks later with persistent pyrexia and abdominal pain localizing to the site of an underlying abscess. Opening or draining the abscess reveals a fecalith that presumably was not removed at prior laparotomy.125
Fistula. Fistulas are created when an inflamed appendix and often an inflammatory mass become adherent to an adjacent organ and ultimately the wall of the adjacent viscus is eroded, causing a fistula. They tend to grow into the skin, including the umbilicus, and adjacent loops of bowel, but may also grow into any adjacent organs including urinary bladder, ureter, ovaries and fallopian tubes. There may be necrotizing fasciitis, and occasionally can even involve vascular grafts.126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137 Rarely, they can be neoplastic (see subsequently).
Suppuration and suppurative pyelophlebitis and hepatic abscess formation. Wound abscesses are relatively common, as are pelvic abscesses. Other sites, particularly the subphrenic space, may also be involved. Suppurative pyelophlebitis is fortunately a rare complication, with pus being present in the veins draining the appendix. In itself it is of little interest, but it is the underlying predisposing factor for hepatic abscesses. Over the last 50 years, appendicitis has changed from being a major cause of liver abscesses to a relatively minor one.138 Nevertheless, it still occurs139, 140, 141 and when present, it still has a very high mortality.
The Appendiceal Stump
Complications involving the appendiceal stump are increasingly seen because appendices are resected laparoscopically and transected rather than being inverted with a purse-string suture.
Recurrent stump appendicitis. This is rare but can occur when appendectomy is carried out without inversion of the appendiceal stump, which is a common practice during laparoscopic appendectomy (see preceding section), so that complications from the stump including leak can cause local abscesses, and it is subject to possible recurrent acute inflammation. It may also occur in patients with multiple appendices, in whom one has been previously removed but acute appendicitis occurs in a second appendix (discussed previously).
Endoscopic excision of the inverted appendix stump. Because the inverted appendiceal stump may appear as a polyp, it may be regarded as a polyp and be resected endoscopically (see Chapter 20).
Abscess formation. This usually occurs about a week following appendectomy. The patient presents with localized or generalized peritonitis. Even more rarely, the stump itself may be associated with necrosis of the cecal wall and subsequent fecal fistula formation. A protracted hospital course, ultimately with recovery, is the usual outcome. Because in laparoscopic appendectomy the stump is not inverted, there is an increased risk of complications such as abscess formation. Staples or suture may be found at the site. This may result in an inflammatory mass in which the omentum may also be involved and wrap itself around the appendix (Fig. 15-20A).
Xanthogranulomatous appendicitis and malacoplakia. These are seen following long-term suppuration, bacterial debris being engulfed by macrophages. The combination of large macrophages and neutrophils is characteristic, and the two are only separated by the presence of small hematoxyphilic bodies in malacoplakia (Michaelis-Gutmann bodies—Fig. 15-20B).
Hemorrhage. Hemorrhage may occur from the appendiceal stump because of the presence of a hemangioma which may be analogous to angiodysplasia.
Increased vascularity in the vicinity of the resected appendix may cause this hemangioma either to develop more quickly than usual or to bleed. It can however be treated by endoscopic diathermy. Less commonly, hemorrhage may be the result of a suture granuloma which goes on to bleed.
Intussusception. Intussusception is rare and usually occurs 2 weeks following appendectomy. Patients present with abdominal pain, vomiting, and possibly rectal bleeding. A mass is palpable in about half of the patients and often requires right hemicolectomy. This is discussed in more detail previously.
Polyps. Polyps, usually serrated polyps (see later), are sometimes detected colonoscopically at the appendiceal orifice. The mistake is to believe that
laparoscopic appendectomy is curative, as the transection invariably occurs distal to the polyp, so will still be there in the appendiceal stump if the patient is recolonoscoped. If the polyp cannot be destroyed colonoscopically, then either ileocolic resection or appendectomy with a cuff of cecum to include the appendiceal orifice is required. In all likelihood the morbidity, and possible mortality, is greater than doing nothing, so the pros and cons of all possibilities need to be considered.
laparoscopic appendectomy is curative, as the transection invariably occurs distal to the polyp, so will still be there in the appendiceal stump if the patient is recolonoscoped. If the polyp cannot be destroyed colonoscopically, then either ileocolic resection or appendectomy with a cuff of cecum to include the appendiceal orifice is required. In all likelihood the morbidity, and possible mortality, is greater than doing nothing, so the pros and cons of all possibilities need to be considered.
SPECIFIC INFECTIONS OF THE APPENDIX
Besides what is currently termed acute idiopathic appendicitis, a variety of specific infections that includes viral, chronic bacterial, fungal, and parasitic infections may affect the appendix. Some of them are associated with clinical appendicitis, albeit relatively infrequently. Infections are discussed more fully in Chapter 19. In the early 1900s the big four recognized causes of appendicitis were tuberculosis, typhoid, amebiasis, and actinomycosis. In some parts of the world these are still encountered, although most pathologists these days can go through their entire career without seeing any of these in the appendix.
Viral Infections
Viral infections in the appendix have been difficult to document even though they have been implicated in the lymphoid hyperplasia sometimes associated with acute appendicitis.142 A variety of viruses has been detected in peripheral blood, omentum, or appendix of children with acute appendicitis.143
Adenovirus. Infection usually occurs in children in the first year of life (Fig. 15-20); it is associated with intussusception, but the intussusceptum is usually hyperplastic lymphoid tissue in the terminal ileum, and the appendix involvement is almost incidental.102, 144, 145 This infection is associated with budding or tufting of the luminal epithelium, and within these tufts, intranuclear changes compatible with viral inclusions can be seen. The virus produces intranuclear eosinophilic inclusions filling nuclei of normal size, so can be quite subtle, but are readily demonstrated immunohistochemically, invariably in attenuated epithelium over lymphoid tissue. They can also make the nuclei appear smudgy and hyperchromatic and hence also referred to as smudge cells.102, 146 There is virtually no differential diagnosis other than reactive changes with prominent nucleoli, as CMV inclusions are primarily seen in the endothelial, stromal, or smooth muscle cells and tend to be much larger, while herpes simplex is undescribed in the appendix.
Cytomegalovirus. CMV appendicitis is invariably part of reactivation and therefore found in patients who are immunosuppressed, especially with HIV/AIDS in those parts of the world where protease inhibitors are not readily available.
Measles. Infection in the form of Warthin-Finkeldey cells (Fig. 15-21) have been found in appendiceal germinal centers of patients incubating measles, as well
as in lymphoid tissue of the oropharynx, although measles is not always the cause.147 Mesenteric nodes may also be enlarged and involved.
as in lymphoid tissue of the oropharynx, although measles is not always the cause.147 Mesenteric nodes may also be enlarged and involved.
Infectious mononucleosis. May also be found incidentally in appendectomy specimens; one report showed the usual proliferation of immunoblasts, including some Reed-Sternberg-like forms, together with a polymorphous lymphoid infiltrate with reactive germinal centers. Similar changes were also present in the mesenteric lymph node.148
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree