In this chapter, solutions and equipment for the various forms of chronic peritoneal dialysis (PD) are described. Apparatus for acute PD is reviewed in Chapter 24.
I. CONTINUOUS AMBULATORY PERITONEAL DIALYSIS (CAPD). In CAPD, dialysis solution is constantly present in the abdomen. The solution is typically changed four times daily, with a range of three to five times, depending on individual patient requirements. Drainage of “spent” dialysate and inflow of fresh dialysis solution are performed manually, using gravity to move fluid into and out of the peritoneal cavity. Technically, PD solution flows into the peritoneal cavity, and dialysate drains out (i.e., the solution does not become dialysate until dialysis has occurred, although the term “dialysate” is commonly used for fresh as well as for used or “spent” solution). In this chapter, the term dialysate is used correctly to refer only to PD solution after it has been instilled into the peritoneal space.
A. Dialysis solutions. CAPD solutions are packaged in clear, flexible plastic bags, typically made from polyvinyl chloride. Some newer PD solutions are packaged with the different solution components in two-chamber (or three-chamber) bags, which are mixed before infusion into the peritoneal cavity.
1. Dialysis solution volumes. For adult patients, CAPD solutions are available in volumes of 1.5, 2.0, 2.25, 2.5, or 3.0 L, depending on the manufacturer. The commonly used bags are routinely overfilled by about 100 mL to allow for flushing, as will be described in a subsequent section. The standard volume prescribed has been 2.0 L, but 2.5 L is also widely used. Generally, larger volumes are prescribed in order to increase solute clearance, but they may not always be tolerated by patients because of symptoms due to the consequent increase in intraperitoneal hydrostatic pressure.
2. Dialysis solution glucose, pH, and glucose degradation products (GDPs). Dextrose (glucose) is the osmotic agent commonly used in CAPD solutions, and preparations containing 1.5%, 2.5%, and 4.25% dextrose (as glucose monohydrate, MW 198) are routinely available and are labeled as such in North America. The true anhydrous dextrose or glucose concentrations (MW 180) in these solutions are 1.36%, 2.27%, and 3.86%, respectively, and this is how they are typically labeled in Europe. The approximate osmolarities of these solutions are 345, 395, and 484 mOsm/L, respectively.
The heat sterilization of glucose leads to generation of glucose degradation products (GDPs), which may have toxic effects both on the peritoneal membrane and systemically. Less GDPs are generated when glucose is heat sterilized at low pH, so in order to minimize GDP generation, the pH of standard lactate-based PD solutions is kept at about 5.5 during heat sterilization. Lowering pH further would decrease GDPs even more, but would also cause infusion pain in patients. A pH of 5.5 on infusion is normally well tolerated, and solution pH rises rapidly as bicarbonate diffuses into the peritoneal cavity from the plasma. However, some patients complain of pain during inflow. This pain can be relieved by neutralizing the dialysis solution pH with alkali prior to instillation.
yThe low pH of PD solution may have adverse effects on leukocytes, impairing their ability for phagocytosis and bacterial killing and may even be harmful to the peritoneal membrane. Therefore, another strategy to reduce GDP generation has been introduced. This is the use of two-compartment solution bags (Fig. 22.1). In one compartment, the glucose is heat sterilized at a very low pH (about 3.2), under which conditions the formation of GDPs is reduced even further. In the other compartment, the rest of the solution is maintained at an alkaline pH during sterilization. At the time of use, the two compartments are allowed to mix, bringing the pH of the resultant combined solution to normal. The end result, therefore, is a low GDP, normal pH PD solution.
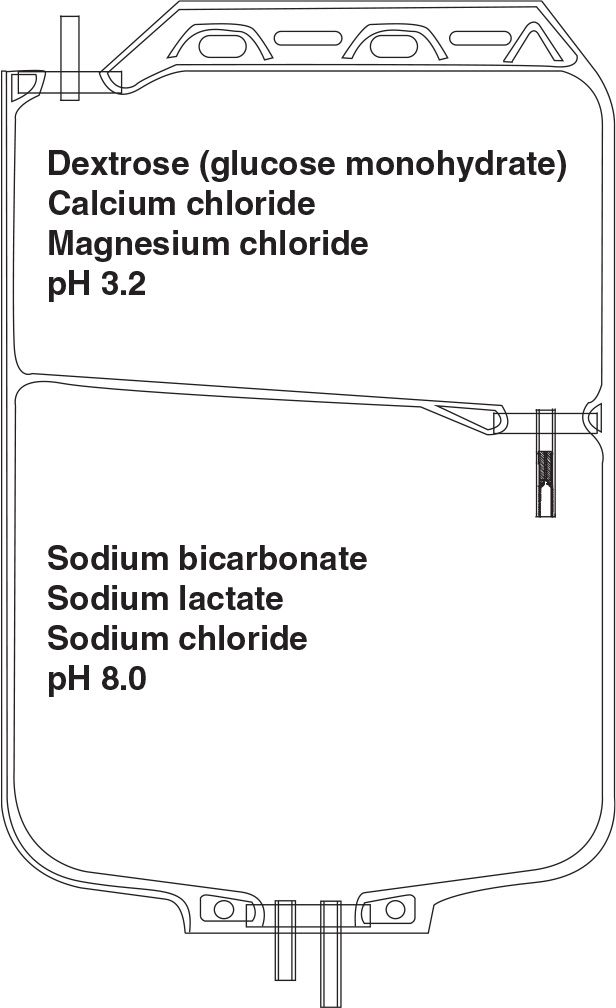
FIGURE 22.1 Two-compartment PD solution bag to allow delivery of a normal pH solution with low GDPs with or without bicarbonate buffer.
3. Dialysis solution buffer and pH. Most commonly marketed PD solutions contain lactate as the bicarbonate-generating base, usually in a 40-mM, or occasionally a 35-mM, concentration. The lactate diffuses across the peritoneal membrane into the bloodstream and is soon metabolized into bicarbonate. A more direct way to supply bicarbonate is to add it directly to the dialysis solution. However, solutions containing bicarbonate and no CO2 have a high pH, at which calcium and magnesium precipitate. For this reason, it is not possible to store bicarbonate-buffered solution in a single-bag system. A variation of the two-compartment-bag system described earlier to limit generation of GDP can be used to enable inclusion of bicarbonate in PD solution. A solution containing calcium and magnesium, a small amount of acid, and other electrolytes is put into one of the compartments, and bicarbonate-containing solution is kept in another compartment. At the time of use, the solutions in the two compartments are mixed together, and the small amount of acid in the calcium/magnesium solution reacts with bicarbonate to generate carbonic acid and CO2, which keeps the pH of the final solution in the physiologic range, at which calcium and magnesium remain in solution. The process is very similar to providing bicarbonate dialysis solution for hemodialysis from a two-component set of concentrates.
Thus, there are at least three commercially available two-bag systems for PD solutions. The Balance solution from Fresenius uses only lactate as the base. The two-bag system is used to limit the formation of GDP by sterilizing the glucose-containing component at low pH. Physioneal, from Baxter, contains both bicarbonate and lactate, and the two-bag system is used both to limit generation of GDP during sterilization and to allow bicarbonate to be used. Bicavera, from Fresenius, contains only bicarbonate and no lactate, and here again, the two-bag system allows use of bicarbonate and also greatly reduced generation of GDP (Table 22.1).
Because these two-bag system solutions are at physiologic or near-physiologic pH after mixing, and because they contain greatly reduced amounts of GDPs, they are theoretically more biocompatible than standard one-bag solutions where the pH is about 5.5. The hope was that these biocompatible solutions would lead to better long-term preservation of peritoneal transport function, including ultrafiltration. It was also hoped that they would enhance peritoneal host defenses and thereby decrease peritonitis rates, and that their use would result in lower serum GDP levels and ultimately better preservation of residual renal function, and that all this would translate into improved technique and patient survival on PD.
There is evidence that each of these dual-bag solutions is effective in dealing with infusion pain. However, this complication occurs only in fewer than 5% of patients using standard low pH PD solutions. With regard to other more important outcomes, results from randomized controlled trials have shown inconsistent results. The recent balANZ study showed a significantly lower peritonitis rate with the Balance solution, but this has not been confirmed in other trials, and a meta-analysis has been negative (Johnson, 2012; Cho, 2014). Some trials have shown better preservation of residual renal function but have also shown less effective ultrafiltration, leading to concern that the improved preservation of renal function with this new solution is simply a consequence of hypervolemia (Davies, 2013). Randomized studies have not been large enough to allow conclusions about long-term patient or technique survival. These biocompatible solutions are widely used in Europe and in parts of Asia but very little in North America and elsewhere, partly because of the lack of consistent high-level evidence supporting their use and partly because of their higher cost.
4. Dialysis solution electrolyte concentrations. The electrolyte concentrations of CAPD solutions vary little by manufacturer. The standard formulations from the three large international manufacturers are shown in Table 22.1. They contain no potassium, and sodium levels are mostly 132–134 mM. Higher sodium concentrations would lead to less diffusive removal of sodium during dwells. Lower sodium solutions have been proposed as a means of augmenting sodium removal, but would require more glucose to maintain a given osmolarity.
With the widespread use of calcium carbonate or calcium acetate as phosphate binders, PD solutions containing 2.0–2.5 mEq/L (1.0–1.25 mM) rather than 3.5 mEq/L (1.75 mM) calcium are increasingly used with the goal of reducing the incidence of the hypercalcemia that is sometimes associated with oral calcium and vitamin D administration. This also protects against adynamic bone disease, which was previously common in PD patients. However, lower calcium PD solutions have been associated with higher plasma parathyroid hormone (PTH) levels. PD solutions typically contain magnesium levels of 1.0 or 0.5 mEq/L (0.5 or 0.25 mM), and this can occasionally result in magnesium depletion.
Commonly Available Peritoneal Dialysis Solution Formulations | |
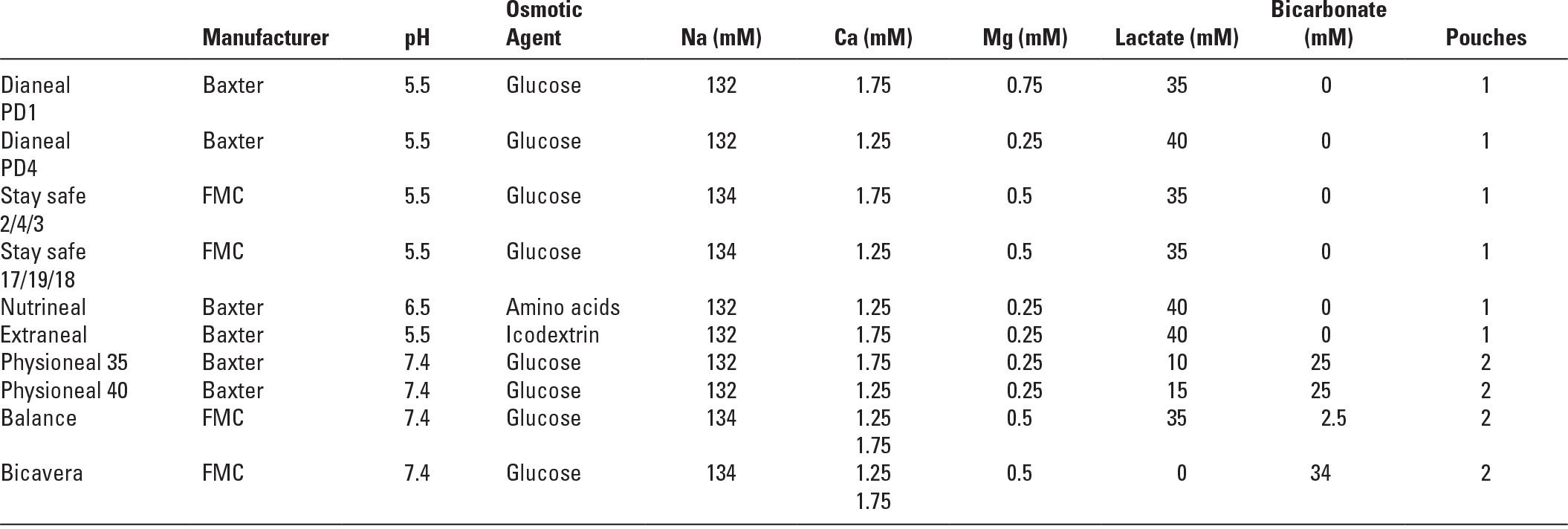
These may differ slightly in name and in formulation from region to region.
All glucose-based solutions are available in three strengths (1.36, 2.27, and 3.86 mg/dL of glucose, equivalent to 1.5, 2.5, and 4.25 mg/dL of dextrose as glucose monohydrate.
To convert calcium from mmol/L (mM) to mg/dL multiply by 4.
To convert magnesium from mmol/L (mM) to mg/dL multiply by 2.43.
FMC, Fresenius Medical Care.
5. Non-glucose solutions. Glucose as an osmotic agent in PD has the advantage of being familiar, relatively safe, and inexpensive, and also is a source of calories. There is concern, however, that instillation of large amounts of glucose into the peritoneal cavity predisposes patients to hyperglycemia, dyslipidemia, obesity, and to long-term peritoneal membrane damage, both directly and via GDPs and the formation of advanced glycosylation end products. Glucose-based solutions are not very effective in high transporters, and inadequate ultrafiltration may result. Alternative osmotic agents are available.
a. Icodextrin. This is a polyglucose solution and is widely used. It is iso-osmolar and induces ultrafiltration by its oncotic effect (Mistry, 1994). Absorption of polyglucose is by the lymphatics and so is much slower compared with glucose. The oncotic effect and the associated ultrafiltration are therefore more sustained than with glucose. For this reason, the main indication to use icodextrin is for the long nocturnal dwell in CAPD and for the long day dwell in automated peritoneal dialysis (APD), especially in patients with ultrafiltration failure. It is generally used for just one dwell daily as it is no more effective than glucose for short dwell times. Icodextrin use is associated with unphysiologic blood levels of maltose and maltotriose, but no associated toxicity has been identified. The increased maltose levels will cause interference with the glucose dehydrogenase pyrroquinolinequinone assay (which reacts with both glucose and maltose) for blood glucose measurements. Therefore, blood glucose should be measured with other methods in patients using icodextrin. In addition, use of icodextrin is associated with mild translocational hyponatremia (owing to movement of sodium-poor fluid from cells to the extracellular fluid). Measured amylase levels can be factitiously low, as a result of interaction between metabolites of icodextrin and commonly used amylase assays.
Icodextrin has been shown in randomized controlled trials to improve ultrafiltration and volume status in PD patients, though not convincingly to reduce blood pressure (Davies, 2003). It has also been shown to improve glycemic control, decrease weight gain, and lessen glucose-induced lipid abnormalities (Cho, 2013; Li, 2013). There is some evidence of better long-term preservation of peritoneal membrane function (Davies, 2005). Disadvantages are its extra cost, occasional skin reactions, and rare sterile peritonitis episodes.
b. Amino acid–based solutions. These are used for nutritional supplementation as they are largely absorbed by the end of a 4- to 6-hour dwell (Jones, 1998). Studies have shown them to be modestly effective in nutritionally compromised patients (Lo, 2003). They are reasonably effective osmotically (comparable to the 1.36% glucose solution) but can be used only once daily because in larger amounts they tend to cause acidosis, as well as a rise in the blood urea. These side effects can be addressed with oral alkali therapy and more dialysis, respectively.
6. Sterility and trace metals. The preparation of PD solutions is carefully regulated to ensure that the final product is bacteriologically safe and has very low concentrations of trace metals.
7. Dialysis solution temperature. PD solutions are usually warmed to body temperature prior to inflow. They can be instilled at room temperature, but uncomfortable lowering of the body temperature and shivering can result. The best warming method is to use a heating pad or special oven. Microwave ovens are frequently used, but this is not recommended by most manufacturers because “hot spots” may be produced during heating, in particular, in the transfer sets. When using a microwave oven, great care must be taken to avoid overheating of the dialysis solution as this can chemically alter the dextrose and may cause discomfort on instillation. Also, accidental boiling of the solution in a confined space may cause an explosion. Heating methods that involve immersing the PD solution container completely in water are also not recommended because contamination can result.
B. Transfer sets. The PD solution bag is connected to the patient’s peritoneal catheter by a length of plastic tubing called a “transfer set” (also sometimes called a “giving set”). There are three major types of transfer sets, each requiring a different method of performing the CAPD exchange. For the purpose of discussion, we will refer to them as the straight transfer set, the Y transfer set, and the double-bag system. Note that some transfer sets are connected to the peritoneal catheter via a short extension or adapter tubing (see what follows).
1. Straight transfer set. This system is now rarely used because it is associated with high rates of peritonitis. However, a brief description is helpful in understanding how more modern systems have evolved.
a. Design. The straight transfer set is a simple plastic tube. One end connects to the peritoneal catheter and the other end to the dialysis solution bag. All exchanges are performed by making and subsequently breaking the connection between the transfer set and the bag. This connection typically involves a spike or a Luer lock.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





