Fig. 21.1
Management algorithm in patients with infectious complications after PNL (Modified from Ref. [50])
21.5.2 Urine and Stone Bacteriology
Mariappan et al. demonstrated that stone and pelvic urine cultures obtained during surgery are better predictors of potential urosepsis than bladder cultures [28]; they found that bladder urine cultures were positive in 11.1 % of cases versus 35.2 and 20.4 % of stone and pelvic urine cultures, respectively. Stone culture showed the greatest PPV (0.7). Infected bladder urine did not always carry identical bacteria to those found in the upper tract. Patients with pelvic- or stone-positive cultures showed a relative risk for urosepsis at least four times greater than the rest of the cohort. In this study, bladder urine did not predict SIRS. Also, they found that preoperative hydronephrosis and stones larger than 20 mm correlated with positive stone and pelvic urine cultures.
Margel and associates [32] obtained similar results. They found different pathogens between bladder urine and stones in 35 % of cases; colonized stones were associated with sterile urine culture in 25 % of patients. The relative risk (RR) of SIRS when the stone culture was positive was 3.6. Dogan and associates [33] also found an increased risk of postoperative fever and sepsis in the group of patients with positive stone and first urine obtained after puncture cultures. They found 35 % of positive stone cultures and 10 % of upper tract urine positive cultures in patients with negative preoperative urine cultures or those who received appropriate antibiotic therapy before surgery. Recently, Lojanapiwat [34] divided 200 patients in two groups, those that presented postoperative SIRS (group I) and those that did not (group II), and found that preoperative urine culture, pelvic urine culture, and stone culture, respectively, were positive in 66.1, 46.4, and 48.2 % of the patients in group I, but only 10.4, 3.5, and 3.5 % for the corresponding specimens in group II. These findings underline the importance of intraoperative microbiologic evaluation of both urine and stones; the obtained cultures may be a guide in the postoperative antibiotic adjustment if a more serious infectious complication develops.
Manipulation of infected stones can cause sepsis due to endotoxemia. McAleer, et al. measured endotoxins levels in renal stones and found markedly higher levels in infection stones [35]. Interaction of bacteria with different intracorporeal lithotripters may have antibacterial effects. In vitro studies have shown a decrease of bacteria viability after use of intracorporeal lithotripsy and laser [36]. Our group has reported recently that extracorporeal shock wave or intracorporeal lithotripsy, using all the alternatives currently available, is significantly effective at reducing the viability of bacteria located inside artificial stone models, including struvite stone models infected with Proteus mirabilis [37–39]. Whether this bactericidal effect is desirable is still to be answered, because reduction in the number of bacteria may represent an increase in the presence of proteins/endotoxins freed from bacterial cell lysis, therefore increasing the risk of urosepsis.
21.5.3 Renal Pelvic Pressure During Surgery
Renal pelvic pressure (RPP) greater than 30 mmHg has been shown to result in pyelovenous and pyelolymphatic backflow. Troxel and Low, in a prospective study that included 31 patients, found that RPP greater than 30 mmHg was recorded only in eight patients (26 %) and did not find any association between RPP levels and postoperative fever [40]. In contrast with them, Zhong et al. demonstrated that mean intrapelvic pressure greater than 20 mmHg and accumulated time of RPP greater than 30 mmHg may cause enough backflow to contribute to bacteremia and postoperative fever [41].
Low RPP during surgery is achieved using an open low-pressure access system such as operating through an Amplatz sheath (operating instrument 4 Ch sizes smaller than the access sheath). Inflow of irrigant should be at gravity and never pressurized. We also recommend the use of forced diuresis (furosemide 20 mg at the beginning of irrigation and every 60 min of surgery and irrigation time) to further reduce the pyelorenal reflux potentially causing fluid overload and bacteremia. Other factors that have been related to postoperative fever and risk of bacteremia are long operative time, large stone burden, and high amounts of irrigating fluid.
In the presence of an obstruction at the ureteropelvic junction or intrarenal segments, purulent urine may be obtained during renal access despite previous negative cultures of the voided urine (Fig. 21.2). In such instances treatment has to be postponed, the urine cultured and the renal collecting system drained under antibiotic coverage, until eradication of the infection is documented.
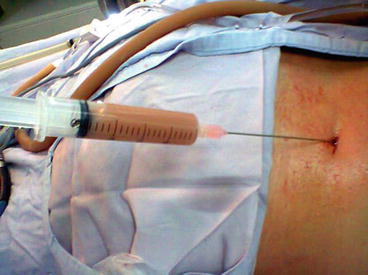

Fig. 21.2
Purulent urine may be obtained during renal access
According to Ramsey et al. in a recent evidence-based review [42], the effects on the resolution of infected hydronephrosis are similar if a ureteral stent or a nephrostomy tube is used. Nevertheless, Mokhmalji et al., in a prospective randomized study, reported prolonged fever and catheter placement time in the group of patients treated with ureteral stent and suggest that percutaneous nephrostomy is superior to ureteral stents for diversion of hydronephrosis [43].
Small case series have been reported, exploring the possibility of continuing the surgery even if purulent urine is incidentally encountered. Aron et al. in a group of 19 patients reported no difference regarding the incidence of postoperative fever or sepsis between patients with one-stage versus staged surgery with collecting system drainage and 3–7 days of intravenous antibiotic coverage before a second procedure [44]. Hosseini et al. divided 45 patients into two groups: in group 1 (n = 29) stones were removed during the first session, and in group 2 (n = 16) a nephrostomy tube remained in place, while stone removal was accomplished 3–5 days later when results of urine and nephrostomy fluid cultures were negative [45]. They reported no intraoperative or postoperative complications, other than transient fever in 10.3 and 12.5 % in groups 1 and 2, respectively.
In spite of these recent reports, there is neither sufficient evidence nor well-designed clinical trials to recommend other conduct than performing a staged procedure with drainage and broad-spectrum antibiotic therapy until infection has resolved.
21.6 Treatment of Complications
21.6.1 Postoperative Fever
Transient postoperative mild to moderate body temperature elevation is frequently seen, is usually secondary to the release of inflammatory mediators, and is not always attributed to an infectious cause [4]. In several studies, discordant rates between postoperative fever and bacteriuria have been reported, ranging from 10–35 % to 0–19 %, respectively [5, 23, 28, 32, 46, 47]. Ziaee and coworkers [48], using three simultaneous laboratory tests including postoperative urine cultures, blood cultures, and postoperative polymerase chain reaction, did not show any difference in bacteriuria between febrile and non-febrile patients. Also, Rao and associates [5] demonstrated a lack of this correlation in their study in which 74 % of patients with PNL had fever postoperatively and only 41 % endotoxemia. These discordances may support the hypothesis that the presence of fever might be the result of inflammatory mediators in response to surgical manipulation rather than infectious in origin. On the other hand, a possible explanation is the inhibitory effect of perioperative antibiotics on bacterial growth.
Thus, in patients with absence of bacteriuria or without struvite stone disease, who are given antibiotics preoperatively and maintained postoperatively, temperature rise usually resolves, does not have clinical significance, and does not necessitate immediate bacteriologic evaluation in those who are hemodynamically stable. Treatment of this group of patients consists of continued antibiotic coverage—i.e., intravenous antibiotics during the hospital stay and oral antibiotics for 5 days after discharge. In these patients, the nephrostomy tube is left to drain 24 h after disappearance of any temperature rise (Fig. 21.1). The risk of a more severe infection and systemic bacteremia is low, provided that appropriate preventive measures are taken.
21.6.2 Persistent Postoperative Fever
Noncontinuous, less than 38 °C persistent postoperative fever in patients without hemodynamic instability should be managed with continuous perioperative oral antibiotics for 5 days (or longer if residual infected stone remains inside the collecting system) and maintaining open nephrostomy tube until the urine is clear. If percutaneous renal drainage is necessary for longer periods (second session planned, status post-ureteropelvic junction repair), urine culture should be revaluated and antibiotic therapy modified or restarted 3 days before any further manipulation, such as antegrade pyelography, repeated treatment sessions, or even clamping of the PNL tube. Prolonged percutaneous renal drainage almost invariably leads to bacteriuria; however, the risk of major infectious complications can be kept to a minimum if these precautions are observed.
21.6.3 Postoperative Sepsis: Early Identification and Initial Treatment
Early recognition and management of sepsis optimizes outcome. Therefore, patients in whom this problem is suspected after genitourinary surgery should be prioritized and receive timely care.
To diagnose sepsis and severe sepsis/septic shock as early as possible, it is necessary to have clear definitions of infection, organ dysfunction, and global tissue hypoxia and to recognize the clinical and laboratory findings that are indicative of these conditions. Sepsis is defined as the presence of SIRS caused by a documented or suspected infection. SIRS is defined as the presence of two or more of the following: (1) temperature greater than 38 °C or less than 36 °C, (2) heart rate greater than 90 beats/min, (3) respiratory rate greater than 20 breaths/min (or PaCO2 <32 Torr), and (4) white blood cell count greater than 12,000/mm3 or greater than 10 % immature band forms. Severe sepsis is defined as the presence of sepsis and one or more organ dysfunctions. Organ dysfunction can be defined as acute lung injury; coagulation abnormalities; thrombocytopenia; altered mental status; renal, liver, or cardiac failure; or hypoperfusion with lactic acidosis. Septic shock is defined as the presence of sepsis and refractory hypotension, i.e., systolic blood pressure less than 90 mmHg and unresponsive to a crystalloid fluid challenge of 500 ml.
As mentioned above, clinical and laboratory recognition of septic problems is mandatory. Procalcitonin is a propeptide of calcitonin, but lacks hormonal activity. During generalized infections with systemic manifestations, its level may rise considerably. In contrast, during severe viral infections or inflammatory reactions of noninfectious origin, procalcitonin levels show no or only a moderate increase. Its exact site of production during inflammatory response is still unknown. The documentation of high levels of early biochemical markers, such as procalcitonin and protein C, in the initial postoperative period may help identify a severe inflammatory response to surgical stress from bacteremia, SIRS, or sepsis/septic shock and prompt the institution of adequate and opportune therapeutic measures [49].
Appropriate therapy is a continuum of infection management ranging from drainage (maintaining indwelling catheter or opening the nephrostomy tube) and broad-spectrum antibiotics to aggressive fluid resuscitation and invasive monitoring with medical management in the intensive care setting, until the causative agent is found and eradicated.
Continuous monitoring of vital signs, pulse oximetry, urine output, and initial laboratory testing to assess the severity of global tissue hypoxia and organ dysfunction, including assessment for lactic acidosis, renal and hepatic dysfunction, acute lung injury, and coagulation abnormalities, should be instituted as soon as possible in patients in whom severe sepsis/septic shock is suspected to facilitate the earliest recognition of this condition.
The usual bacteria cultured from urinary sources are aerobic Gram-negative bacilli and enterococci. Appropriate cultures (including blood and urine) should be obtained before the adjustment of antibiotics. At this point, it is important to reanalyze urine cultures that were obtained preoperatively or during surgery and, based on their results, redirect antibiotic therapy. If results are not available, empiric broad-spectrum antibiotics should be initiated as soon as possible. Suggested primary regimens include the usage of ampicillin/gentamicin, or piperacillin–tazobactam, or carbapenems (doripenem, imipenem, or meropenem). The duration of treatment is determined by the patient’s clinical response. It is imperative to modify the antibiotic regimen to a culture directed one when possible. If severe sepsis/septic shock is recognized, besides empiric antibiotic therapy, prompt treatment in the intensive care unit should include repletion of intravascular volume with large amounts of crystalloid intravenous fluids. Pressors are administered as needed to maintain blood pressure, central venous pressures are monitored, and fluids are administered to maintain a pressure of 8–12 cm H2O. Bicarbonate and low-dose steroids may be used and good blood glucose control maintained. Tight blood glucose control by administration of insulin doses up to 50 U/h is associated with a reduction in mortality. Recombinant activated protein C (drotrecogin alfa) is a new drug that has been approved for therapy of severe sepsis. Multidisciplinary treatment is essential to obtain good results [12, 50, 51].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








