The rectum and anus are two anatomically complex organs with diverse pathologies. This article reviews the basic anatomy of the rectum and anus. In addition, it addresses the current radiographic techniques used to evaluate these structures, specifically ultrasound, magnetic resonance imaging, and defecography.
Key points
- •
The rectum and anus are two anatomically complex organs with diverse pathologies.
- •
The focus of this article is a review of the basic anatomy of the rectum and anus.
- •
In addition, we also address the current radiographic techniques used to evaluate these structures, specifically ultrasound, MRI, and defecography.
Embryology
The embryo begins the third week of development as a bilayered germ disk. During week 3 the surface facing the yolk sac becomes the definitive endoderm; the surface facing the amniotic sac becomes the ectoderm. The middle layer is called mesoderm. The long axis and left-right axis of the embryo also are established at this time. The buccopharyngeal membrane marks the oral opening; the future openings of the urogenital and the digestive tracts become identifiable as the cloacal membrane. At 4 weeks of gestation, the alimentary tract is divided into three parts: (1) foregut, (2) midgut, and (3) hindgut. The primitive gut results from incorporation of the endoderm-lined yolk sac cavity into the embryo, following embryonic cephalocaudal and lateral folding. The endoderm gives rise to the epithelial lining of the gastrointestinal tract while muscle, connective tissue, and peritoneum originate from the splanchnic mesoderm. Development progresses through the stages of physiologic herniation return to the abdomen, and fixation. The acquisition of length and formation of dedicated blood and lymphatic supplies takes place during this time. With folding of the embryo during the fourth week of development, the mesodermal layer splits. The portion that adheres to endoderm forms the visceral peritoneum, whereas the part that adheres to ectoderm forms the parietal peritoneum. The space between the two layers becomes the peritoneal cavity. Foregut-derived structures end at the second portion of the duodenum and rely on the celiac artery for blood supply. The midgut, extending from the duodenal ampulla to the distal transverse colon, is based on the superior mesenteric artery. The distal third of the transverse colon, descending colon, and rectum evolve from the hindgut fold and are supplied by the inferior mesenteric artery. Venous and lymphatic channels mirror their arterial counterparts and follow the same embryologic divisions. At the dentate line, endoderm-derived tissues fuse with the ectoderm-derived proctodeum, or ingrowth from the anal pit. Two major theories exist to explain the differentiation of the hindgut into the urogenital (ventral) and anorectal (dorsal) part: the theory of the septation of the cloaca; and the theory of the migration of the rectum. In the initial stages of development the hindgut is in continuity with the midgut cranially; caudally, it is in direct contact with the ectoderm. As development progresses, the caudal part of the hindgut, the cloaca, differentiates into two separate organ systems: the urogenital tract and the anorectal tract. The normal development of these tracts depends on the proper differentiation of the cloaca by the urorectal septum. Anorectal separation from the urogenital structures occurs as a result of either a cranially orientated septum growing down to reach the cloacal membrane and fuse with it or from lateral folds encroaching on the lumen of the cloaca from either side and fusing in the middle, or from a combination of the two processes.
Embryology
The embryo begins the third week of development as a bilayered germ disk. During week 3 the surface facing the yolk sac becomes the definitive endoderm; the surface facing the amniotic sac becomes the ectoderm. The middle layer is called mesoderm. The long axis and left-right axis of the embryo also are established at this time. The buccopharyngeal membrane marks the oral opening; the future openings of the urogenital and the digestive tracts become identifiable as the cloacal membrane. At 4 weeks of gestation, the alimentary tract is divided into three parts: (1) foregut, (2) midgut, and (3) hindgut. The primitive gut results from incorporation of the endoderm-lined yolk sac cavity into the embryo, following embryonic cephalocaudal and lateral folding. The endoderm gives rise to the epithelial lining of the gastrointestinal tract while muscle, connective tissue, and peritoneum originate from the splanchnic mesoderm. Development progresses through the stages of physiologic herniation return to the abdomen, and fixation. The acquisition of length and formation of dedicated blood and lymphatic supplies takes place during this time. With folding of the embryo during the fourth week of development, the mesodermal layer splits. The portion that adheres to endoderm forms the visceral peritoneum, whereas the part that adheres to ectoderm forms the parietal peritoneum. The space between the two layers becomes the peritoneal cavity. Foregut-derived structures end at the second portion of the duodenum and rely on the celiac artery for blood supply. The midgut, extending from the duodenal ampulla to the distal transverse colon, is based on the superior mesenteric artery. The distal third of the transverse colon, descending colon, and rectum evolve from the hindgut fold and are supplied by the inferior mesenteric artery. Venous and lymphatic channels mirror their arterial counterparts and follow the same embryologic divisions. At the dentate line, endoderm-derived tissues fuse with the ectoderm-derived proctodeum, or ingrowth from the anal pit. Two major theories exist to explain the differentiation of the hindgut into the urogenital (ventral) and anorectal (dorsal) part: the theory of the septation of the cloaca; and the theory of the migration of the rectum. In the initial stages of development the hindgut is in continuity with the midgut cranially; caudally, it is in direct contact with the ectoderm. As development progresses, the caudal part of the hindgut, the cloaca, differentiates into two separate organ systems: the urogenital tract and the anorectal tract. The normal development of these tracts depends on the proper differentiation of the cloaca by the urorectal septum. Anorectal separation from the urogenital structures occurs as a result of either a cranially orientated septum growing down to reach the cloacal membrane and fuse with it or from lateral folds encroaching on the lumen of the cloaca from either side and fusing in the middle, or from a combination of the two processes.
Rectal anatomy
The rectum is the terminal segment of the large intestine. It begins proximally at the rectosigmoid junction and extends approximately 12 to 15 cm where it terminates at the level of the levator ani. The rectosigmoid junction is identified intra-abdominally as the point where the distinct taenia coli of the colon splay onto the anterior surface of the rectum, with subsequent absence of distinct taenia coli on the rectum. Furthermore, the haustra ( Fig. 1 ) that are prominent throughout the colon are no longer apparent at the proximal portion of the rectum.
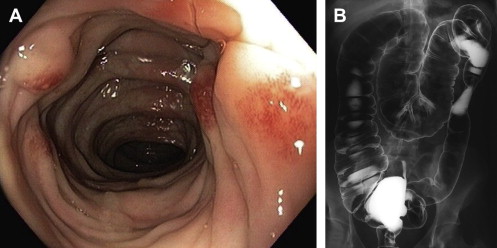
Identifying the proximal and distal aspects of rectum becomes more difficult when viewed endoscopically. The most consistent landmarks are the valves of Houston. The valves of Houston are folds of rectum that do not contain all layers of the rectal wall and have no known physiologic function. Most commonly, there are three valves, but the number and location of these valves is variable. The uppermost valve is usually seen on the left lateral wall of the rectum at 8 to 16 cm from the anal verge. The middle valve is located 7 to 12 cm from the anal verge and is on the right lateral wall of the rectum. Finally, the most distal fold is at 5 to 10 cm from the anal verge and is found on the left lateral rectal wall. Of the three valves, the middle valve is most consistent and is closely associated with the peritoneal reflection, and is also referred to as Kohlrausch plica.
The blood supply of the rectum comes mainly from the superior hemorrhoidal artery. The superior hemorrhoidal is a direct extension of the inferior mesenteric artery and perfuses the proximal two-thirds of the rectum. The middle hemorrhoidal artery is generally described as a branch of the internal iliac artery and travels anterolaterlaly, to supply the lower one-third of the rectum, but its course and origin are highly variable. The inferior hemorrhoidal artery supplies mainly the anus, but does perfuse the rectum by an extensive submucosal network of vessels.
The venous drainage of the rectum mirrors that of the arterial supply. The superior rectal vein drains into the inferior mesenteric vein and subsequently the portal circulation. The middle rectal vein drains to the internal iliac vein, which then flows to the inferior vena cava, thereby bypassing the portal circulation.
Like the venous drainage, the lymphatic system mirrors the arterial supply. The upper two-thirds of the rectum drains along the superior hemorrhoidal artery to inferior mesenteric nodes and then para-aortic nodes. The lower one-third of rectum drains both superiorly along the superior hemorrhoidal distribution, and laterally, following the middle hemorrhoidal artery to a nodal basin along the internal iliac artery.
The rectum is surrounded by an investing fascia propria that envelopes the rectum, perirectal fat, and a plexus of blood vessels. The tissues surrounding the rectum that are bound by the fascia propria are commonly referred to as the mesorectum. The mesorectum contains many perirectal lymph nodes that can harbor nodal metastases in rectal cancer. Given this, recognizing and preserving the fascia propria during a proctectomy for cancer is essential for performing an adequate cancer operation. Posterior to the rectum and fascia propria is the presacral fascia. This lining separates the rectum from the presacral venous plexus. Again, it is critical to identify and avoid injury to this fascial layer operatively, because injury to the underlying venous plexus can cause brisk bleeding that is often very difficult to control. As the presacral fascia descends further into the pelvis (S2-S4), it fuses with the posterior mesorectal fasica forming a reflection known as the rectosacral fascia, or Waldeyer fascia. Anteriorly, the peritoneum extends along about two-thirds of the rectum. Just caudal to the peritoneal reflection is the visceral pelvic fascia or Denonvilliers fascia. This fascial covering separates the mesorectum from the prostate and seminal vesicles in men, and the vagina in women. Again, this is an important anatomic landmark in proctectomy to avoid injury to important structures while also preserving an intact mesorectum.
Lying in the plane between the fascia propria and the presacral fascia are the pelvic nerves. Innervation to the rectum is composed of sympathetic and parasympathetic components. The sympathetic nerves branch from the sympathetic trunk at L1-L3 and synapse at the preaoritc plexus. Some postsynaptic nerves travel directly to the upper rectum to supply sympathetic innervation. Other nerves travel distally and form the hypogastric plexus. This plexus extends laterally along the rectum and forms a second, more distal pelvic plexus. They continue on distally and course between the mesorectum and presacral plexus. Nerves from the hypogastric and pelvic plexus supply sympathetic innervation to the lower rectum.
The parasympathetic nerves originate from S2-S4 and exit the spinal canal by the sacral foramina and join the hypogastric and pelvic plexus. This group of parasympathetic nerves is also known as the nervi erigentes. These nerves then travel postsynaptically in a proximal direction to send parasympathetic innervation to the distal colon and upper rectum. They also travel directly to the lower rectum and anus. Both sympathetic and parasympathetic fibers travel anterior and form a plexus of nerves along Denonvilliers fascia, known as the periprostatic plexus in men. This group of nerves is much harder to identify and can be injured if the operative plane of dissection is too lateral.
The sympathetic and parasympathetic fibers coursing along the rectum are also responsible for innervation of the remainder of the pelvic organs, and thus essential for sexual function. In men, sympathetic innervation is responsible for emission of semen, whereas parasympathetic innervation is responsible for ejaculation. Given this, injury to proximal sympathetic nerves with preservation of the nervi erigentes results in retrograde ejaculation. As one travels distally, the nervi erigentes become threatened, particularly at the level of the lateral rectal stalks. Injury at this level leads to a loss of erectile function and ejaculation. Finally, dissection anterior to the rectum threatens the periprostatic plexus, with injury here also resulting in loss of erectile function and bladder dysfunction. In women, the role of specific nerves in sexual function is not well understood, but dysfunction resulting from nerve injury during pelvic dissection is well documented. Following pelvic dissection the most commonly reported symptoms of sexual dysfunction are dyspareunia and decreased lubrication during intercourse.
Anal anatomy
The anus is a short, yet complex structure at the terminal end of the gastrointestinal tract. It is grossly composed of the anal canal, anal verge, and anal margin. Surrounding these structures is a group of muscles that are essential for maintenance of fecal continence, and injuries to this intricate area can lead to devastating consequences.
The anal canal is anatomically defined as extending from the dentate line to the anal verge. Functionally, however, the accepted boundaries extend from the proximal aspect of the internal anal sphincter (IAS)/levator ani muscle to the anal verge, a length of approximately 4 cm. The anal canal includes the dentate line and is surrounded by the IAS and external anal sphincter (EAS).
The dentate line represents an important landmark, because the blood supply and innervation of the anal canal transition at this point. Proximal to the dentate line, the anus is innervated by sympathetic and parasympathetic nerves, with no somatic pain fibers. However, distal to the dentate line, the anal canal has somatic innervation. The dentate line itself is identified by the anal valves. These valves are extensions of anal glands, with ducts carrying mucous from gland into valves. These ducts course outward from the anal valve, originating at anal glands either in the IAS or in the intersphincteric groove between the IAS and EAS. Knowledge of this anatomy is important because it helps to explain the location of perianal abscesses.
The anal verge is a narrow band of tissue that separates anal canal from perianal skin. It extends from the intersphincteric groove onto the skin surrounding the anus. It is covered with thin squamous epithelium that is easily identified because it lacks hair follicles (anoderm). The distinct border between anal verge and perianal skin, as identified by the appearance of hair follicles and keratinized epithelium, is the anal margin. This anatomic landmark is important to recognize, because it is frequently used as the external reference point for proximal lesions or pathology.
The muscles surrounding the anal canal provide for continence. They can be separated into the IAS, EAS, and the levator ani. Each plays an important role in maintaining continence, with injury to the muscle or nervous supply to each component causing varying degrees of problems.
The IAS is a direct extension of the inner circular muscle layer of the rectum. It extends distally just beyond the EAS and approximately 1 cm beyond the dentate line. The space between the IAS and EAS is the intersphincteric groove, and can be usually palpated on digital rectal examination. The IAS is smooth muscle, and subsequently innervated with sympathetic nerves from L5 and parasympathetic nerves from S2-S4, with a state of tonic contraction that contributes to baseline continence.
The EAS is wrapped radially around the IAS, extending the length of the anal canal and terminating just proximal to the IAS. It is “suspended” around the anus, with the anococcygeal ligament anchoring it anteriorly to the perineal body and posteriorly to the coccyx. Although it has been divided into multiple units (deep vs superficial), it functions as a single muscular unit and is best thought of as such. Unlike the IAS, the EAS is composed of skeletal muscle and is innervated by branches of the pudendal nerve and the perineal branch of S4. The EAS is also in a constant state of tonic contraction, but also has a component of voluntary control. During episodes of threatened continence, the EAS can increase its contractile strength to avoid fecal or gaseous incontinence.
The levator ani, with the proximal aspect of the EAS, marks the proximal extent of the anal canal. It is a broad, thin sheet of three defined muscles that form the pelvic floor. The puborectalis muscle forms a sling around the anal canal, with anterior attachments on the pubis. It is found just cephalad to the EAS and intimately associated with the anal canal. Essentially, this sling pulls the anal canal anteriorly, forming the anorectal angle, another component of fecal continence. The other two muscles comprising the levator ani, the pubococcygeus and iliococcygeus, form the major component of the pelvic floor. The pubococcygeus fans out from posterior attachments to the anococcygeal raphe, with anterior attachments along the length of the pubis, forming the main anterior part of the pelvic floor. The posterior aspect of the pelvic floor is comprised of the coccygeus muscle that also extends from bony attachments to S3, S4, and the coccyx to the anococcygeal raphe.
Although the muscular function of the IAS and EAS contribute to full continence, the sensory function of the anal canal also plays an important role. The upper anal canal has an abundance of nerve endings to help with sensation of stool and gas in the lower rectum. Furthermore, there are nervous plexuses that are responsible for sensation of pressure, touch, and temperature that also contribute to maintenance of continence. These nerves travel by the pudendal nerve to the central nervous system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







