, Ivan Damjanov1 and Ryan M. Taylor2
(1)
University of Kansas School of Medicine, University of Kansas, Kansas City, KS, USA
(2)
Division of Gastroenterology and Hepatology, University of Kansas School of Medicine, Kansas City, KS, USA
Liver tumors may be classified as benign or malignant. The malignant tumors may be classified as primary or metastatic. Figure 1 illustrates a standardized diagnostic approach to these lesions.
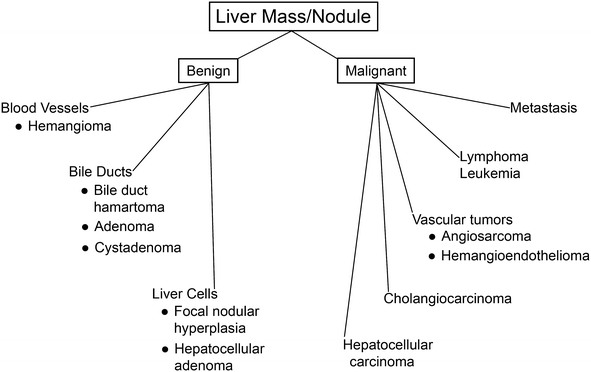

Fig. 1.
Diagnostic approach to liver lesions
The following facts about tumors of the liver are worth remembering:
Primary liver tumors may originate from hepatocytes, bile ductular epithelium or endothelial cells lining the sinusoids or hepatic vessels.
Overall the most common tumors of the liver are hemangiomas, which are found in approximately 5 % of all persons.
The most common primary malignant tumors are hepatocellular carcinomas (HCC) arising in a background of underlying cirrhosis.
The incidence of HCC shows marked geographic variation and is highest in east Asia and Africa.
Metastases are much more common than primary malignant liver tumors.
Non-neoplastic lesions such as bile duct hamartomas and focal nodular hyperplasia (FNH) must be distinguished from true neoplasms.
Hepatocellular Adenoma
Hepatocellular adenomas (HCA) are benign tumors composed of liver cells resembling normal hepatocytes (Dhingra and Fiel 2014). HCA are more common in females than males, and are linked to oral contraceptive use, estrogen intake as well as exogenous androgen use (Torbenson 2015). Obesity and some inborn errors of metabolism (e.g. glycogenosis I and IV) are other risk factors.
PATHOLOGY. Neoplastic hepatocytes resemble normal liver cells (Fig. 2). Tumor cells form plates which are somewhat thicker than in the normal liver (2–3 cell thick). Like in the normal liver cells the plates are enclosed into a well developed reticulin framework. HCA are usually not encapsulated, or only partially encapsulated and may have irregular borders. They lack internal nodularity and do not contain portal tracts or fibrosis. HCAs receive blood through “unpaired arteries”, which differ from portal branches of hepatic artery by not being paired with bile ducts (Fig. 3). These unpaired arteries are a hallmark of HCAs and particularly useful if spotted in a slender needle biopsy specimen. HCA are usually solitary but may be multiple. If there are more than ten nodules the condition is called adenomatosis (Frulio et al. 2014).
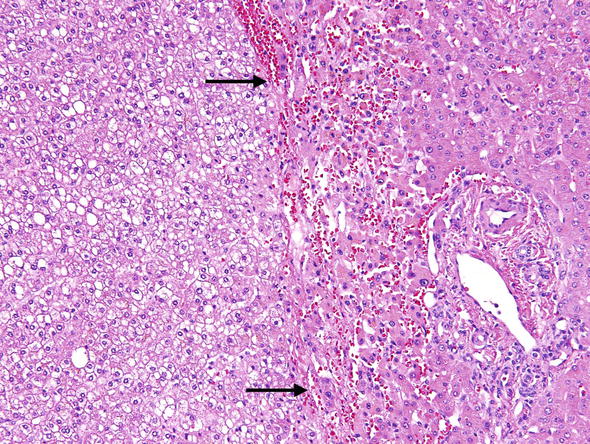
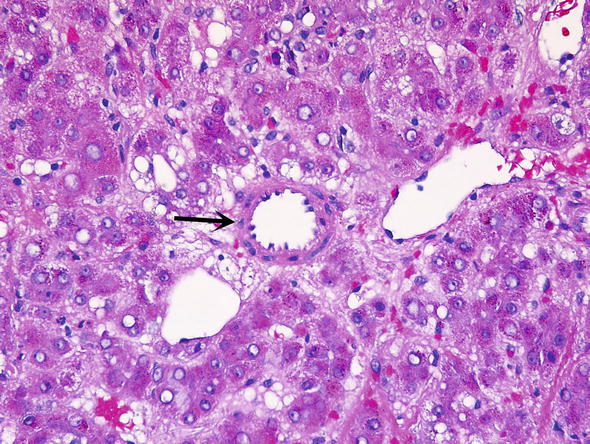

Fig. 2.
Hepatic adenoma. The neoplastic cells of hepatocellular adenomas resemble normal liver cells. The border between neoplastic and non-neoplastic liver is demarcated by the arrows. On the right is normal liver showing a portal tract. On the left is the adenoma, which does not show portal tracts

Fig. 3.
Hepatic adenoma. Unpaired muscular arteries (arrow) are the hallmark of hepatic adenomas and an important clue to neoplastic tissue in the liver
Molecular biology of HCA has been studied in great detail and these data are used to support the histologic classification of HCA (Sempoux et al. 2013; Nault et al. 2013). Four histologic subtypes of HCA have been identified based on mutations in specific oncogenes and tumor suppressor genes and are recognized as follows:
I.
HNF1A inactivated HCA. Tumors that have inactivated hepatocyte nuclear factor 1 alpha (HNF1A) represent 30–40 % of adenomas. These tumors may occur in families, and are often associated with maturity-onset diabetes type 3 (MODY3). They are usually diagnosed in younger patients. These tumors are characterized by marked steatosis, a lack of cytologic atypia, and no inflammatory infiltrates (Fig. 4). The risk of transforming into hepatocellular carcinoma (HCC) or being associated with and HCC is approximately 7 %.

Fig. 4.
HNF-α mutated adenoma. HNF-α mutated adenoma have marked steatosis (asterisk), little cytologic atypia and minimal inflammation. The border between adenoma and background liver is demarcated with arrows
II.
β-Catenin mutated HCA. These tumors comprise 10–15 % of adenomas and are more common in men. β-Catenin-activated mutations are exclusive of HNF1A mutations. About half of these tumors are also inflammatory. These adenomas are characterized by cytologic abnormalities and pseudo-glandular structures and nuclear β-catenin expression by immunohistochemistry. These tumors have the highest risk of transformation to HCC, at approximately 40–50 %.
III.
Telangiectatic/Inflammatory HCA. This group of adenomas historically was considered as a subtype of focal nodular hyperplasia until it was shown that they have monoclonality. They represent the most common subtype and account for approximately 40–50 % of all HCA. The cardinal feature of inflammatory adenoma is the activation of the JAK/STAT pathway. They lack HNFA1 and β-catenin mutations and are more common in women than in men. The microscopic characteristics of telangiectatic/inflammatory HCAs includes hepatocellular proliferation with hepatic plate atrophy, sinusoidal dilatation (often with marked vascular ectasia), aberrant naked arteries, abortive portal tracts, and some degree of inflammation and ductular reaction (Fig. 5). The ductular reaction can be subtle and is best visualized immunohistochemically with the antibody to cytokeratin 7 (CK7) (Fig. 6). This subtype carries little if any risk of malignant transformation. Telangiectatic/inflammatory HCAs are prone to hemorrhage and are best managed by surgical excision.
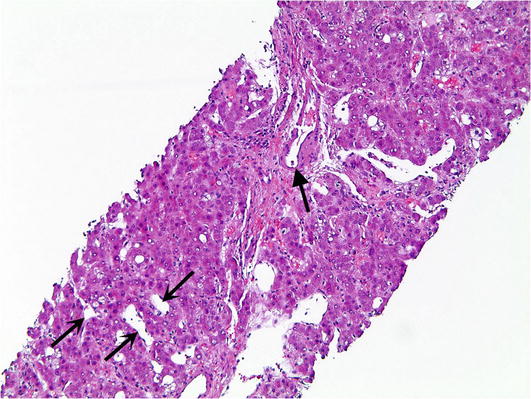
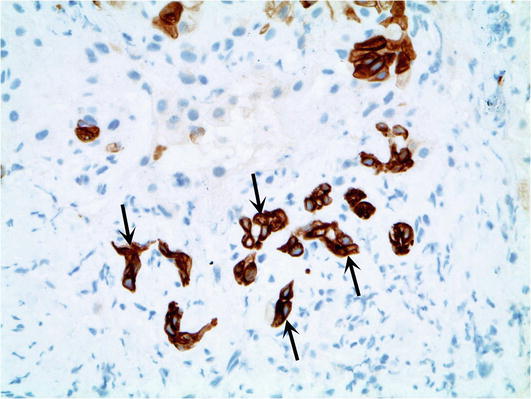

Fig. 5.
Telangiectatic/Inflammatory hepatic adenoma. Telangiectatic/Inflammatory hepatic adenomas show unpaired arteries (closed arrow) and sinusoidal dilatation (open arrows)

Fig. 6.
Telangiectatic/Inflammatory hepatic adenoma. The poorly formed ductules associated with telangiectatic/inflammatory hepatic adenoma are highlighted by cytokeratin 7 immunohistochemical stain
IV.
Other forms of HCA. This group includes all HCAs that cannot be classified otherwise. They are considered non-mutated and non-inflammatory and have a 13 % risk of malignant transformation.
Differential Diagnosis
Focal nodular hyperplasia. In contrast to HCA, these benign lesions contain a central fibrous scar and more fibrosis in general, which is usually associated with bile ductular proliferation at the connective tissue/hepatocyte interface. Glutamate synthetase immunohistochemical reaction stains focal nodular hyperplasia unevenly in a “geographic pattern” (Fig. 7), in contrast to HCA which are either completely negative or diffusely positive (in β-catenin positive HCA). Critical use of immunohistochemistry has been found to be useful in clinical pathologic practice (Bioulac-Sage et al. 2014).
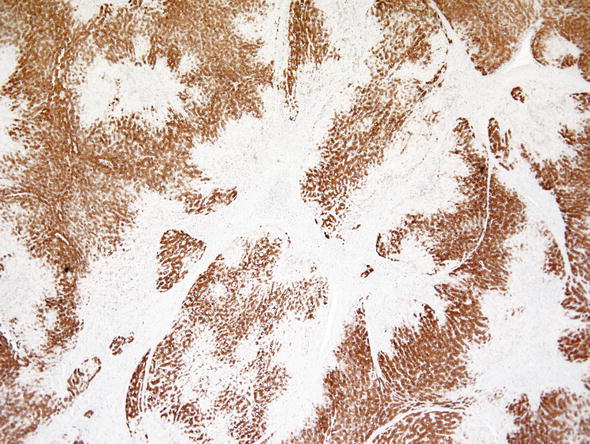
Fig. 7.
Focal nodular hyperplasia. Glutamine synthetase shows a geographic map-like pattern in focal nodular hyperplasia
Hepatocellular carcinoma. In contrast to HCA, tumor cells of hepatocellular carcinoma show prominent nuclear atypia and nucleoli. There are mitoses and areas of necrosis. Glypican-3 immunohistochemistry gives positive results in hepatocellular carcinoma, whereas HCA is negative. However, in well differentiated hepatocellular carcinomas glypican-3 may be not expressed. The same holds true for alpha-fetoprotein which is usually expressed in poorly differentiated hepatocellular carcinomas, but not in HCA and well differentiated hepatocellular carcinomas.
Comments
1.
HCA present radiologically as well vascularized lesions and the CT scanning and MRI provide additional data that are useful for the diagnosis and triage of these lesions. The identification of specific molecular pathways, largely contributed by authors from Bordeaux and Paris, France, has led to a better understanding of benign hepatocyte neoplasms. Molecular biology data have been translated into diagnostic and prognostic algorithms which promote personalized clinical care. Nault et al. (2013) have compiled detailed algorithms for the diagnosis and treatment of FNH and HCAs combining clinical, radiologic, pathologic and molecular data. This algorithm includes treatment recommendations that are stratified by the risk of complications (hemorrhage and malignant transformation for HCA and the absence of complications for FNH).
Focal Nodular Hyperplasia
Focal nodular hyperplasia (FNA) is a non-neoplastic nodular lesion that most often occurs in young women, but may be found in men as well (Belghiti et al. 2014). FNA develop due to disturbances in blood flow. They are usually solitary but may be multiple as well. Treatment of larger and symptomatic lesions includes resection, but small FNHs and those that cause no symptoms do not require surgical treatment (Navarro et al. 2014).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








