Anatomy of the Epididymis, Vas Deferens, and Seminal Vesicle
AKANKSHA MEHTA
It is well known that spermatogenesis, the development of germ cells into structurally mature sperm, occurs in the seminiferous tubules of the testis through a series of ordered and well-regulated processes. Less well appreciated is the fact that testicular sperm are functionally immature and therefore incapable of fertilizing an oocyte without direct intracytoplasmic injection (ICSI). Sperm maturation occurs as sperm migrate out of the testis and travel through the male reproductive tract. In their journey, they are supported and stimulated by the distinct microenvironments of the epididymis and vas deferens and bathed in the secretions of the prostate, seminal vesicles, and accessory glands before eventually exiting the male urethra during ejaculation. Physiologic changes that sperm undergo during migration include plasma membrane remodeling, changes in intracellular signaling transduction, and the acquisition of progressive motility, all of which contribute to ultimately rendering spermatozoa capable of oocyte penetration and fertilization. The unique anatomy of the excurrent ductal system, both gross and microscopic, is optimally suited to ensure that sperm maturation progresses in as efficient and as effective a manner as possible. An appreciation of this anatomy is key for understanding not only normal male reproductive physiology but also male factor infertility.
EPIDIDYMIS
Physical Examination
Careful assessment of the epididymides during the physical examination of an infertile patient is as important as the testicular evaluation. The size and consistency of the epididymis can help differentiate between obstructive and nonobstructive etiologies of azoospermia. An enlarged and indurated epididymis suggests obstruction of sperm outflow, whereas a flat epididymis suggests impaired spermatogenesis. Pain upon palpation of the epididymis is concerning for inflammation or infection due to chemical, viral, or bacterial causes, depending on the patient’s history and risk factors. Palpation of a cystic structure originating from the caput epididymis is suggestive of a spermatocele, a diagnosis that is confirmed by documenting the presence of sperm in the aspirate. Although rare, solid tumors originating from the epididymis have been described (1). These may be primary or secondary, benign or malignant, and warrant surgical excision. Absence of the distal epididymis on physical examination should alert the clinician to the possibility of ipsilateral absence of the vas deferens, which, when noted bilaterally, should prompt an evaluation for cystic fibrosis.
Development
The development of the epididymis from the wolffian or mesonephric duct occurs between the 8th and 12th week of gestation under the influence of testosterone produced by fetal Leydig cells (2,3). Specifically, the proximal portion of the wolffian duct differentiates into the epididymis, whereas the distal portion gives rise to the vas deferens. As the cranial end of the wolffian duct degenerates, it leaves behind a small remnant of tissue called the appendix epididymis. During the 9th week of gestation, 5 to 12 nephric ducts in the region of the epididymis make contact with the developing sex cords of the future rete testis. It is not until the third month of gestation, however, that these tubules establish a communication with the rete testis as the efferent ductules (2,4). In humans, the epididymis is well developed by 16 weeks of gestation, and secretory activity of the epididymal cells has been observed by 25 weeks of gestation (5).
The major morphogenic event in epididymal development is the elongation and coiling of the wolffian duct, a process that is likely the combined product of cell proliferation and directed cell rearrangements, and interactions between wolffian duct epithelium and surrounding mesenchymal cells (6).
Anatomy
The epididymis lies posterior and slightly lateral to the testis. It is invested by the tunica vaginalis, which is continuous with the tunica vaginalis covering the testis. Laterally, a deep groove, termed the sinus epididymis, marks the boundary between the testis and epididymis. Extensions from this connective tissue sheath enter the interductal spaces, forming septa that divide the tubule into histologically similar regions (7).
Antoine De Graaf, the famous 17th century anatomist, reported his dissection of the human epididymis in 1668, likening the epididymis to a “thin thread which gradually enlarges until it attains the thickness of a piece of string and constitutes the vas deferens” (8). It is estimated that the uncoiled human epididymis measures between 6 and 7 meters in length.
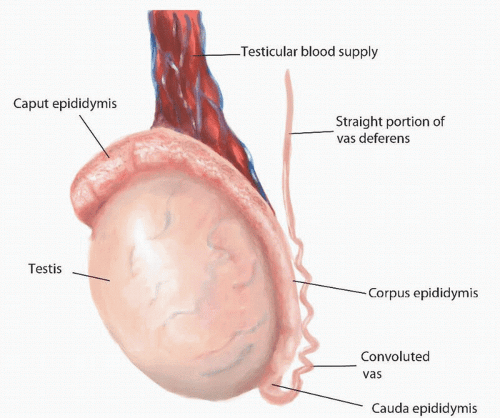
Anatomically, the epididymis is divided into three regions: the caput (head), corpus (body), and cauda (tail) (Fig. 49.1).
The caput epididymis is formed by 8 to 12 efferent ducts that originate from the superior pole of the testis and drain into the epididymis. Each efferent duct is 15 to 20 cm in length and opens into an individual epididymal tubule. The epididymal tubules anastomose with one another along the length of the epididymis, eventually becoming a single, coiled tubule in the corpus epididymis. The epididymal tubules are surrounded by contractile smooth muscle cells, which increase in size and number in the distal epididymis. Peristaltic contractions generated by the smooth muscle propel the spermatozoa in an antegrade direction. At its distal end, the cauda epididymis becomes continuous with the convoluted portion of the vas deferens. Here, contractile cells are replaced by smooth muscle organized in three layers—inner longitudinal, intermediate circular, and outer longitudinal—that thicken and continue into the vas deferens.
The caput epididymis is formed by 8 to 12 efferent ducts that originate from the superior pole of the testis and drain into the epididymis. Each efferent duct is 15 to 20 cm in length and opens into an individual epididymal tubule. The epididymal tubules anastomose with one another along the length of the epididymis, eventually becoming a single, coiled tubule in the corpus epididymis. The epididymal tubules are surrounded by contractile smooth muscle cells, which increase in size and number in the distal epididymis. Peristaltic contractions generated by the smooth muscle propel the spermatozoa in an antegrade direction. At its distal end, the cauda epididymis becomes continuous with the convoluted portion of the vas deferens. Here, contractile cells are replaced by smooth muscle organized in three layers—inner longitudinal, intermediate circular, and outer longitudinal—that thicken and continue into the vas deferens.
The luminal shape and size on cross section of the epididymis varies from the irregular stellate shape in the efferent ducts to the regular, oval, or round shape in the corpus epididymis to the wide irregular shape in the cauda epididymis. Variability in luminal appearance correlates with the different functions performed by each segment of the epididymis and with the different cell types found along its length.
The lumen of the epididymis is lined by nonciliated pseudostratified columnar epithelium and contains four major cell types: principal, basal, apical, and clear cells. Principal cells are tall, columnar cells with basally located, oval nuclei. They bear long stereocilia (15 µm) and function to resorb testicular fluid; approximately 90% of the total secretory fluid volume is absorbed in the epididymis. Principal cells also endocytose other components of seminal fluid and produce glycoproteins that are essential for sperm maturation. Basal cells lie between the principal cells, at their base, and are thought to be precursors of principal cells. Apical and clear cells are far less common than principal and basal cells. Apical cells are rich in mitochondria and most abundant in the caput epididymis. In contrast, clear cells are columnar and most abundant in the caput epididymis. They have few microvilli but numerous endocytic vesicles and lipid droplets. The exact functions of apical and clear cells are unknown.
The composition of epididymal fluid varies along the length of the epididymis, giving rise to several distinct microenvironments within the tubule (9). These are maintained by the active secretion of proteins by the epithelium, fluid reabsorption, and exchange of molecules between the lumen and the circulation. Exchanges are selectively regulated by the blood-epididymis
barrier, which is formed by tight apical junctions between adjacent principal cells (10).
barrier, which is formed by tight apical junctions between adjacent principal cells (10).
The delicate structure of the epididymis makes it vulnerable to iatrogenic injury during procedures such as hydrocelectomy, spermatocelectomy, and orchidopexy, especially if the epididymis is adherent to the tunica vaginalis (11). Additionally, trauma, infection, inflammation, and even prolonged obstruction of the vas deferens, as, for example, after vasectomy, can cause an increase in intraluminal pressure, leading to microscopic ruptures with sperm leakage, sperm granuloma formation at the site of the rupture and, consequently, secondary obstruction of the epididymis.
Blood Supply
The primary arterial supply to the epididymis derives from the testicular artery, with small contributions from the deferential and cremasteric arteries. The testicular artery (also called the internal spermatic artery) arises from the abdominal aorta and courses inferolaterally, under the parietal peritoneum and along the psoas major, toward the pelvis. As the right and left testicular arteries enter the pelvis, they lie anterior to the genitofemoral nerves, ureters, and external iliac arteries. Both arteries then enter the deep internal inguinal ring and travel with the ipsilateral spermatic cord in the inguinal canal to the scrotum. In its course to the testis, the testicular artery branches into an internal artery and an inferior testicular artery and into branches supplying the caput, corpus, and cauda epididymis (12). The level at which this branching occurs is variable; in 31% to 88% of cases, it occurs within the inguinal canal (13,14).
The deferential artery is a branch of the superior (and occasionally inferior) vesical artery, which, in turn, arises from the internal iliac (hypogastric) artery. The cremasteric artery (also called the external spermatic artery) is a branch of the inferior epigastric artery, which accompanies the spermatic cord and supplies the cremaster muscle and other coverings of the cord and the tunica vaginalis surrounding the testis. Both the deferential and the cremasteric arteries enter the inguinal canal at the deep inguinal ring and travel the length of the spermatic cord along with the testicular artery and veins within the scrotum. Rich vascular anastomoses occur at the head of the epididymis, between the testicular and epididymal arteries, and at the tail of the epididymis between the testicular, epididymal, cremasteric, and deferential (vasal) arteries.
Innervation
Sympathetic nerve endings are concentrated in the corpus and cauda epididymis and increase in density in the proximity of the vas deferens, consistent with their role in promoting epididymal contractility. During ejaculation, the distal portion of the epididymis demonstrates intermittent contractions, in contrast to the peristaltic activity of the proximal epididymis (15).
Function
The mean transit time for sperm in the human epididymis is approximately 12 days (16). In addition to providing sperm storage and facilitating sperm transport, the epididymis plays an important role in sperm maturation. Here, spermatozoa undergo a number of physiologic changes, rendering them capable of oocyte penetration and fertilization within the female reproductive tract. The majority of these changes have not been reproduced in vitro, indicating that their triggers have an epididymal origin. Importantly, sperm maturation within the epididymis has been shown to be androgen-dependent (17).
Human spermatozoa acquire an increased capacity for motility as they migrate through the epididymis, which is manifested not only as a quantitative increase in the percentage of spermatozoa with motile tails but also as a qualitative change from an immature to a more mature pattern of motility. Spermatozoa in the proximal epididymis demonstrate high-amplitude, low-frequency tail movements, producing little motion. In contrast, spermatozoa in the cauda epididymis, demonstrate low-amplitude and high-frequency tail movements, resulting in considerably greater forward progression (18). However, the extent to which motility is dependent of the interaction of spermatozoa with a particular section of the epididymis is unknown. In patients with congenital absence or obstruction of the vas deferens, spermatozoa in the distal epididymis demonstrate worse motility compared to spermatozoa in the proximal epididymis (19,20). Therefore, intrinsic sperm processes and the epididymal microenvironment both appear to play an important role in the acquisition of progressive sperm motility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree