This article reviews anatomic considerations in penile and urethral surgery, with a particular emphasis on lymphatic anatomy. The historical evolution of techniques to reduce the unnecessary use and operative morbidity of inguinofemoral lymph node dissection are reviewed, with an emphasis on sentinel node (SN) biopsy and dynamic SN biopsy techniques.
Although penile and male urethral cancers are 2 of the least common urologic malignancies, they are important for the urologist to identify and diagnose promptly and treat adequately for several reasons. First, diagnosis is often delayed because of embarrassment on behalf of the patient in seeking treatment or delayed diagnosis on account of the treating physician because they are rare and can manifest with a wide array of presentations and symptoms. Second, complete surgical excision is the only therapy that is potentially curative, even in the case of node-positive disease. These 2 cancers are often accompanied by substantial local symptoms that can prove difficult to control if the disease is allowed to progress without surgical intervention.
The crux of the penile cancer dilemma for the urologist is that diagnostic modalities for identifying nodal disease must demonstrate near-perfect sensitivity. This demonstration is difficult because clinical examination is notoriously inaccurate, with 11% to 62% of negative examinations demonstrating micrometastatic disease at the time of inguinofemoral lymph node (LN) dissection (IFLND). Moreover, preemptive IFLND has been shown to demonstrate a survival advantage over delayed IFLND in T2N0M0 disease or T1 tumors with unfavorable characteristics (high grade, lymphovascular invasion), suggesting that missing an early diagnosis of micrometastatic disease may result in a lost opportunity for cure. Failed detection and treatment of microscopic node-positive disease has been demonstrated to result in only 35% survival at 3 years. Therefore, in the case of patients with intermediate- or high-risk disease and nonpalpable nodes, the ultimate goal would be to develop a diagnostic tool that will identify patients with micrometastatic disease to the groins with high sensitivity and low morbidity.
Although historical treatment of primary urethral cancer has been primarily surgical, emerging data suggest that multimodal therapy may be a reasonable alternative for some patients with invasive urethral cancer. Unlike penile cancer, prophylactic IFLND has not resulted in a survival benefit for patients with metastatic urethral cancer.
This article reviews the anatomy relevant to the diagnosis and management of penile and urethral cancer, as well as the historical development of diagnostic modalities for the assessment of inguinal nodes.
Anatomic considerations: penis, blood vessels, and integuments
Anatomically, the penis is divided into the root, the body, and the glans, and is structurally composed of 2 smooth muscle-containing erectile bodies and the surrounding integuments ( Fig. 1 ). The erectile bodies are distinct proximally, where they course inferior to the pubic rami, and distally, where they separate to form 2 distinct corporal tips that terminate in the glans. However, along the length of the body of the penis, these 2 structures freely communicate through a shared, perforated midline septum. The urethra is similarly divided into the posterior (prostatic-membranous) and anterior (bulbar-pendulous-glanular) urethra. The bulbar and pendulous urethra is surrounded by a third erectile body, the corpus spongiosum. Distally, the corpus spongiosum fuses to form the glans.
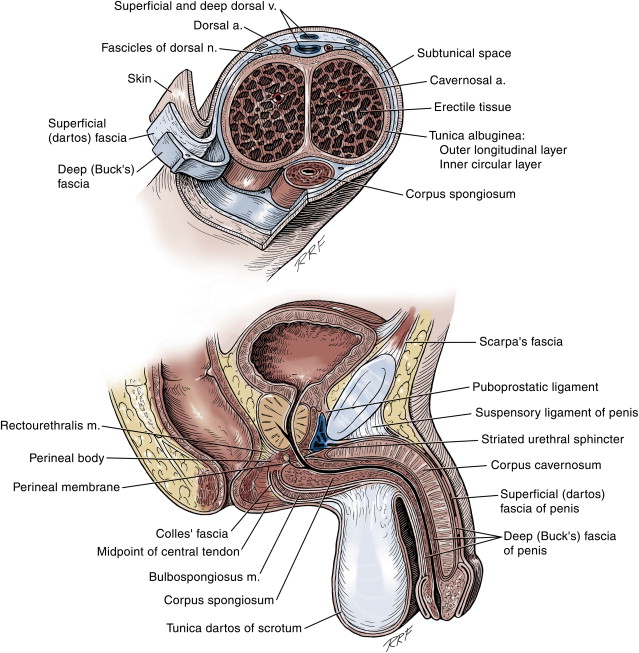
The posterior urethra is believed to develop from the urogenital sinus, the anterior separation of the cloaca after descent of the urorectal septum at a gestational age of approximately 5 weeks. Although anterior urethral development remains controversial, most investigators agree that the anterior urethra is derived by medial migration of mesodermal pilings that develop lateral to the cloacal membrane as it extends toward the genital tubercle. These structures develop into the penile and proximal glanular urethra. Complete development of the distal urethra, frenulum, and circumferential prepuce depends on ventral migration of the urethral and preputial folds. The distally located lacunar folds fuse to form the distal one-third of the glanular urethra, which is lined only by ectoderm. All these infoldings occur in a carefully orchestrated sequence in a proximal-to-distal fashion. Development of the corpora cavernosa occurs via coalescence of the mesoderm that flanks the urethral groove dorsolaterally and is thought to be secondary to urethral development, possibly acting as an inductive force on development of the erectile bodies. Although the exact mechanism that drives penile (corporal and urethral) elongation has not been elucidated, paracrine testosterone production plays an important role in this process.
The penis is enveloped in 2 layers of fascia. The superficial fascia is continuous with the dartos fascia and connected to the underlying structures by loose areolar tissues, making this the plane that separates easily for penile degloving. The deep fascia (Buck’s fascia and the tunica albuginea of the corpus cavernosae) comprises the dense fibrous layer that surrounds the erectile chambers and allows for expansion in length and girth with stimulation to the elastic limit of the tissues, thus providing rigidity for erections. The deep fascia of the root of the penis is continuous with the external oblique fascia and fascia of the urogenital diaphragm.
Arterial supply to the penis is well described, and anatomic variability is often present. The main blood supply, the common penile artery, arises from the internal pudendal artery, a branch of the internal iliac artery. The paired common penile arteries trifurcate into the paired bulbourethral arteries, cavernosal arteries, and dorsal penile arteries. The bulbourethral arteries enter the bulbar urethra posteriorly, near the upturn of the most dependent portion of the bulbar corpus spongiosum. The cavernosal arteries course through the middle of the corpora cavernosa and are responsible for arterial inflow during initiation of erections. The dorsal penile arteries course under Buck’s fascia to feed the corpus spongiosum in a retrograde fashion via the glans. The arteries also send off circumflex branches to the corpora spongiosum and corpora cavernosa. Together, intact bulbourethral and dorsal penile arterial supply guarantee redundant blood supply to the anterior urethra, allowing transection of the urethra and reanastomosis. The most common and surgically relevant variation to arterial blood supply is the possible presence of accessory pudendal arteries. These arteries occur approximately 30% of the time and are most often branches either of the obturator (84%) or other iliac branches. The skin of the penis is supplied by branches of the external pudendal arteries, which are branches of the proximal femoral arteries.
Penile and urethral venous drainage consists of the superficial, intermediate, and deep systems. The superficial drainage system comprises multiple veins that run in a dorsolateral fashion between Colles’ fascia and Buck’s fascia. These superficial branches coalesce into a single or paired superficial dorsal vein that subsequently drains into one or both saphenous veins. The intermediate system originates as emissary veins from the glans to form the retrocoronal plexus, which subsequently drain into the deep dorsal vein. Along the way, circumflex veins (arising from the corpus spongiosum) and distal emissary veins (arising from the corpus cavernosum) further contribute to the deep dorsal vein. The deep dorsal vein may be single or multiple and subsequently courses between the limbs of the suspensory ligament to contribute to the dorsal vein complex of the prostate. The deep venous system consists of the cavernous and crural veins. The cavernous veins run along the dorsum of the urethral bulb under the crus of the penis and drain into the internal pudendal system. There are multiple connections between this system and the periprostatic plexus. The crural veins emerge from the dorsolateral surface of the penis and drain into the internal pudendal veins.
The major sensory and somatic supply to the penis is derived from the pudendal nerve (S2–4). After passing through the Alcocky’s canal, the pudendal nerve sends a dorsal branch to the penis as the dorsal nerve of the penis. This branch is believed to be responsible for the penile and glanular sensory apparatus. The dorsal nerve pierces the transversus perinei muscle and travels along the dorsum of the penis toward the glans, lateral to the dorsal arteries. The nerve’s course is relatively invariable. After leaving the pudendal canal, the pudendal nerve terminates as 2 branches: the inferior rectal and perineal branches. The inferior rectal nerve courses posteriorly to the external anal sphincter. The perineal nerve has been demonstrated to provide motor function to the bulbospongiosus muscle and then pierce this muscle along its midline raphe to innervate the corpus spongiosum. These perineal nerve branches course along the urethra laterally and intermingle with branches of the dorsal nerve of the penis distally. The cavernous nerves are a network of fine fibers that emanate from the corpora cavernosa and course along with the cavernous artery and vein, deep to the dorsal vein complex of the prostate and along the prostatic capsule as part of the prostatic neurovascular bundle.
Anatomic considerations: lymphatic drainage
Penile Lymphatics
Penile lymphatic drainage parallels venous drainage, with a superficial system that drains the skin and a deeper system that drains the glans and corporal bodies ( Fig. 2 ). The lymphocapillary networks originate in the skin, in the mucous membrane and submucosa of the urethra, in the septum of the glans, in the tunica albuginea of the corpora cavernosa, and in the fascia. The skin of the glans is composed of a bilayered network located in the stratum papillare and the stratum reticulare, and there is free communication between the 2 networks. The networks running parallel to the skin are oriented radially from the urethral meatus. All these radially oriented branches coalesce to form a single network at the corona, occupying the papillary and reticular layers of the skin, and course through the inner and outer prepuce. The skin of the penile shaft is more segregated, with distinct layers of lymphatics coursing in the papillary and reticular layers of the skin. A separate lymphatic system is seen in the fascia of the penis and tunica albuginea.
Cadaver dissections of the inguinal regions suggest that the superficial lymphatic basin for the penis is bounded superiorly by a line 1 cm above and parallel to the inguinal ligament, beginning medially just above the pubic tubercle (over the adductor canal) and coursing for a length of 12 cm. The inferior boundary is marked by a perpendicular line dropped 20 cm from the lateral extent of this line and 15 cm from the medial extent. The superficial inguinal nodes were divided by Rouviere into 5 zones (superomedial, superolateral, inferomedial, inferolateral, and central) ( Figs. 3 and 4 ). This area contains 4 to 25 LNs (mean = 8.25). The deep inguinal lymphatic basin is smaller in size and is located primarily along the medial aspect of the femoral vessels deep to the fascia lata ( Fig. 5 ).
Cabanas described the lymphoangiographic patterns demonstrated in a large series of patients with penile cancers (N = 80) and benign diseases of the penis (N = 10). Injection into the lymphatics of the dorsal penile vessels consistently drained to a LN located anterior or medial to the superficial epigastric vein and superomedial to the epigastric-saphenous junction, with subsequent drainage into the deep inguinal and iliac chains. All patients in this study who subsequently went on to have IFLND and who were found to have metastatic disease demonstrated involvement of this sentinel node (SN). No prepubic LNs were identified. However, subsequent investigators have challenged these findings, reporting that the location of or drainage to the SN was not as consistent as that reported initially by Cabanas. Although inguinal drainage patterns still remain somewhat contested, it is almost universally accepted that penile lymphatics drain to the inguinal nodes before draining into the iliac nodes. Anecdotal observations of patients with positive iliac metastases in the setting of negative inguinal dissections have been reported. Such a presence is likely caused by undersampling of the inguinal nodes either at the time of dissection or at the time of pathologic assessment.
Urethral Lymphatics
Urethral lymphatic drainage rests in a double-layered lymphocapillary network that runs parallel to the urethra, is situated partially in the mucous membrane and partially in the submucosa, and undulates with the folds of the urethral wall. This network is particularly dense in the region of the fossa navicularis. These smaller branches coalesce into 4 main collecting trunks. Distally, these lymphatics traverse the urethra at the level of the frenulum and join the penile lymphatics of the glans at the prepuce. The penile urethral lymphatics course laterally around the corpora cavernosae and join the lymphatic trunks from the glans. Bulbar urethral drainage is more variable with some lymphatics coursing along the bulbar artery, some terminating in the medial retrofemoral node, and others coursing superiorly under the pubis along the dorsum of the prostate toward the anterior bladder wall, terminating in the retrofemoral nodes and medial external iliac nodes. The lymphatic drainage of the prostatic urethra corresponds to lymphatic drainage of the prostate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




