Primary tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
T1
Tumor 2 cm or less in greatest dimension
T2
Tumor more than 2 cm but not more than 5 cm in greatest dimension
T3
Tumor more than 5 cm in greatest dimension
T4
Tumor of any size that invades adjacent extradermal tissue(s): bone, cartilage, skeletal muscle
Regional lymph nodes (N)
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Regional lymph node metastasis
Distant metastases (M)
MX
Distant metastasis cannot be assessed
M0
No distant metastasis
M1
Distant metastasis
Until 40 years ago, treatment of anal cancer usually consisted of abdominoperineal resection. The standard of care for epidermoid anal cancer is now concurrent chemoradiotherapy with surgery usually reserved for those with residual disease following systemic therapy or locoregional recurrence [9]. Even in patients with locally advanced anal cancer with infiltration of the prostate or vaginal fistulization, the first-line treatment is chemoradiotherapy. In those with a vaginal fistula and associated fecal incontinence, a defunctioning stoma may be necessary for symptomatic control. The timing of repeat biopsies of the anal canal lesion is important. We recommend waiting 10–12 weeks following final dose of radiotherapy before performing EUA and biopsy. In patients who have undergone chemoradiotherapy and are found to have persistent local disease or develop recurrent disease, then a salvage abdominoperineal resection is recommended. In this scenario an extralevator abdominoperineal resection is critical. We would perform the abdominal component of the procedure in the lithotomy position and the perineal part with the patient in the prone-jackknife position. In these cases we would consider the application of a myocutaneous flap to the dead space. These perineal wounds have a high potential for breakdown as a significant part of the perianal and or perineal skin often has to be removed leaving a dead space best filled by autologous tissue. Whether one used a gracilis, rectus abdominis of gluteal flap depends on patient factors and opinion/experience of the plastic reconstructive surgeon involved in the case.
Often following chemoradiotherapy, patients complain of significant anorectal dysfunction. This includes tenesmus, bowel frequency, and urge and true fecal incontinence. Radiation proctitis with associated rectal bleeding is often a nuisance reducing quality of life (Fig. 12.1). Treatment options consist of topical medications such as steroid and sucralfate enemas. Other treatments include the use of formalin solution or endoscopic argon plasma coagulation (APC) [10]. While APC has a limited depth of penetration, we would advise caution given potential for a devastating rectovesical or rectourethral fistula especially when applied in the prostatic region. In refractory cases we have used hyperbaric oxygen therapy, and on occasions, significantly symptomatic patients have resorted to colostomy. Infertility is also a significant risk in both sexes.


Fig. 12.1
Radiation proctitis with characteristic telangiectasia
If a diagnosis of adenocarcinoma is histologically confirmed, it should be treated as a rectal cancer (neoadjuvant chemoradiotherapy or short-course radiotherapy and anterior resection/abdominoperineal resection depending on tumor location). If a perianal skin cancer can be safely removed with a clear margin without sphincter compromise, then this is appropriate with careful follow-up. If the perianal skin cancer is extensive or there is risk of sphincter damage with excision, then chemoradiotherapy is appropriate. However, there is still significant debate as to what represents a true perianal skin canal and what is a true anal canal cancer. If the perianal skin lesion encroaches on the anal verge, then we tend to treat it as a true anal canal lesion given potential for lymph node involvement.
In addition to endorectal ultrasound, MRI, and CT scans, there has been interest of late in 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography (PET). Its value, however, is questionable because most tumors demonstrate some element of uptake even following treatment, and the consensus is that PET tends to overstage anal cancer. Thus, its role in the detection of locoregional disease in patients with biopsy-proven anal canal caner is still evolving [11].
Common presentations of anal canal cancer are rectal bleeding (often attributed to hemorrhoids which may coexist), pain on defecation, palpable mass, or tenesmus. Almost a quarter of patients have no symptoms at the time of diagnosis, and anal cancer is an incidental finding. Irrespective of age, one should have a high index of suspicion in patients presenting with bleeding per rectum and avoid the presumption that it is due to hemorrhoids. Investigations should be performed in stepwise manner.
History and clinical examination
Full colonoscopy
+/−Examination under anesthesia with biopsy
+/−CT abdomen/pelvis
Surgeons and physicians dealing with anorectal pathology should be familiar with the potential differential diagnoses:
Hemorrhoid
Anal skin tag
Squamous cell carcinoma of the anus
Adenocarcinoma of the rectum
Condylomata acuminata
Malignant melanoma
The following cases highlight some of the diagnostic and management dilemmas that may be associated with management of anal cancer. They represent typical patients commonly presenting to any colorectal service. A short case history is followed by a brief description of learning points and discussion of evidence-based management options where applicable.
12.3 Case 1
A 43-year-old heterosexual man with no history of sexually transmitted infections presented to the colorectal department with a small verrucous lesion close to the anal canal. He complained of local pain, intermittent abscess formation, pruritus, bleeding, malodor, and altered bowel habits. He underwent full colonoscopy which was otherwise normal and was booked for wide local excision of the lesion. Unfortunately, he failed to attend for the operation. Three years later, he represented with a far larger lesion (14 × 9 cm) and a slightly smaller lesion on the contralateral anal canal. MRI scan confirmed that there was no deep invasion. He underwent EUA and biopsy. Histology confirmed verrucous carcinoma .
12.3.1 Learning Points
Associated with HPV-6 and HPV-11, verrucous carcinomas or Buschke–Lowenstein tumors are large, soft, fleshy, painful, cauliflower-like cancer.
They are slow growing but relentless.
Although benign, they have potential for local erosion to the ischioanal fossa and perirectal tissue. They do not metastasize.
Microscopically, verrucous carcinoma looks like condyloma acuminata. Wide local excision is the treatment of choice, with abdominoperineal resection (APR) performed in unusual cases of late-stage disease with sphincter invasion.
Radical excision may be necessary in order to achieve a cure.
Reports of radiotherapy, imiquimod treatment, and CO2 laser treatment are available, but wide excision with postoperative vigilance looking for recurrences is the mainstay of therapy.
12.4 Case 2
A 39-year-old homosexual male presented with anal discomfort, intermittent bright red blood per rectum, tenesmus, and a palpable mass inside the anal verge. He had had unprotected anal intercourse with multiple partners over the preceding 10 years.
Examination under anesthesia (EUA) revealed a hard pedunculated mass below the dentate line. Multiple biopsies were taken and sent for urgent histopathology. Further examination demonstrated enlarged, hard, non-tender lymph nodes in the left inguinal canal.
Endoanal ultrasound was performed for evaluation of tumor size and extension and infiltration of the sphincter muscle complex. It confirmed a 4 × 2 cm lesion invading the anal sphincter (T3N1). Biopsies demonstrated invasive squamous cell carcinoma. He was found to have HPV p16 infection. P63 positivity confirmed the squamous origin of the tumor (Fig. 12.2a–c). Staging CT of thorax, abdomen, and pelvis was negative for distant metastases. Serology was positive for human immunodeficiency virus (HIV). CD4 count was 620.


Fig. 12.2
(a) Invasive squamous cell carcinoma . (b) Immunohistochemistry with p16 showing diffuse staining in invasive squamous cell carcinoma indicating association with high-risk HPV. (c) Immunohistochemistry positivity with p63 nuclear stain confirming “squamous” origin of the carcinoma
Chemoradiotherapy and antiretroviral therapy were commenced. EUA was performed 12 weeks following completion of systemic treatment, and there was no visible or palpable lesion. Biopsies were taken and did not demonstrate any residual tumor cells. The patient was considered to have undergone a complete pathological response and was enrolled on a surveillance program to ensure early detection in the case of recurrent disease.
12.4.1 Learning Points
The relation between anal cancer and receptive anal intercourse is similar in both sexes and is independent of immunosuppression [12].
Anal cancer is much more frequent in HIV-positive homosexual males in comparison with HIV-seronegative persons [13]. All high-risk patients presenting with anal cancer should undergo HIV testing.
Presentation of anal cancer in the HIV-positive population does not differ when compared to the general population. Anal pain, fissures, fistulae, diarrhea, bleeding, and exophytic verrucous anal lesions are the most common clinical manifestations [14].
The combination of HAART and chemoradiotherapy is as safe and effective for immunodeficient and immunocompetent patients [12–15].
Pretreatment CD4 count <200/mm3 increases the likelihood of toxicity, and so these patients should be treated with caution, while patients with CD4 counts ≥200/mm3 can be expected to tolerate combined modality therapy.
12.5 Case 3
A 55-year-old woman presented with perianal discomfort and a sensation of irregular skin while cleaning with intermittent “spotting” of bright red blood. She was previously fit and healthy apart from a hysterectomy 20 years earlier for cervical cancer. Local and distant staging confirmed a T3N1 squamous cell cancer of the anal canal, and she underwent chemoradiotherapy (45 Gy in 1.8-Gy fractions over 5 weeks) with 5-FU (1000 mg/m2 per day on days 1–4 and 28–31) and bolus mitomycin (10 mg/m2 on days 1 and 28, with a maximum of 20 mg per cycle) [16]. She had a good clinical response and tolerated therapy well. Examination under anesthesia was performed 12 weeks following completion of chemoradiotherapy. No abnormality was detected either clinically or histologically. At surveillance EUA 18 months later, she was found to have a lesion at the site of the previous tumor (Fig. 12.3a). Biopsy confirmed it to be a squamous cell carcinoma. Following discussion at the multidisciplinary meeting (MDM), the decision was taken to perform a salvage abdominoperineal resection with posterior vaginectomy. A gracilis flap was used. Final histology demonstrated a T3N0M0 tumor (Fig. 12.3b, c). She made an uneventful recovery and will have surveillance CT scans to monitor for distant metastases.


Fig. 12.3
(a) Recurrent anal canal cancer . (b) Low power of ulcerating residual tumor bed anal canal. (c) H&E section showing focal residual carcinoma where residual tumor cells show a glandular-type morphology (mucin globules and gland outline)—a well-recognized phenomenon in post-therapy changes. Near-complete response to neoadjuvant therapy (immunohistochemistry confirmed squamous immunophenotype as did review of pretreatment biopsy)
12.5.1 Learning Points
There is no consensus as to whether physical examination alone is sufficient following treatment or whether biopsy is necessary in the presence of complete clinical response.
Five-year local disease failure rates are different between those patients receiving radiation alone (52.5 %) and those receiving combined chemoradiation (35.3 %). In the radiation alone group, age, total radiation dose <50 Gy, and higher T stage predicted local failure. For patients receiving combined chemoradiation, no predictive factor was identified [17].
The preferred treatment for persistent disease following combined modality therapy is APR. The main complications of this procedure are delayed wound healing, wound infection, and local failure [18]. Unfortunately wound complications are common in all previously irradiated skin.
Salvage chemoradiotherapy has been described, but up to a third of these patients inevitably ultimately require APR [16].
Any patient with a history of cervical cancer should be carefully evaluated for anal cancer because of the causative role of HPV in both [17].
12.6 Case 4
A 39-year-old man presented for day-case hemorrhoidal artery ligation (HAL) for treatment of hemorrhoids. He was a vague historian but complained of perianal itch, occasional mucus discharge, and intermittent bright red bleeding per rectum. He was a smoker with a previous history of genital warts. He was placed in the lithotomy position, and an examination under anesthesia was performed. The hemorrhoid at the 7 o’clock position appeared unusual. It was large, bulky, and polypoid in nature. The operating surgeon decided not to proceed with HAL but to take multiple biopsies of the unusual hemorrhoid. Histology confirmed a poorly differentiated squamous cell carcinoma of the anus (Fig. 12.4a, b). Local and distant staging were undertaken, and the patient underwent chemoradiotherapy for management of anal cancer. He had an excellent clinicopathological response.
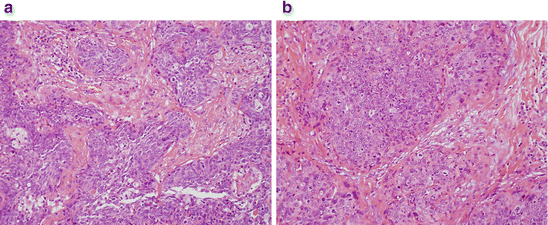

Fig. 12.4
(a) Invasive nonkeratinizing squamous cell carcinoma . (b) Abundant mitoses and cell pleomorphism indicating poorly differentiated tumor
12.6.1 Learning Points
A low index of suspicion is essential when dealing with perianal pathology, and any unexpected findings should be thoroughly investigated to rule out neoplasia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








