- A variety of tumor types can arise in the anal canal and surrounding perianal skin; squamous cell tumors are the most common.
- Infection with human papillomavirus is strongly associated with development of squamous cell anal tumors.
- Treatment of anal canal cancer has evolved from abdominoperineal resection to sphincter-preserving, “definitive” concurrent radiation therapy and chemotherapy in most cases.
- A number of ongoing clinical studies will continue to refine the combined modality approach to squamous cell anal cancer in an effort to improve tumor outcomes while minimizing toxicity.
US National Comprehensive Cancer Network—provides guidelines for diagnosis, staging, and management of various cancer
American Society of Clinical Oncology—provides resources on cancer education and policies
- Different types of tumors can arise in the anal canal. Treatments are tailored to the histology, with concurrent radiation therapy and chemotherapy usually the treatment of choice in localized squamous cell tumors, with surgery reserved for local tumor persistence or recurrence. Surgery can play an important role in the treatment of anal canal adenocarcinomas.
- Concurrent radiation therapy and chemotherapy can provide long-term disease-free survival in the management of squamous cell anal cancer, and requires close monitoring of acute and late toxicities.
Epidemiology
Prior to reviewing the epidemiology of anal cancer, one must first understand the anatomical relations and histological features of the “anal canal.” A variety of terms regarding anatomical classification and histology of the anal canal are present in the literature, which can lead to some confusion.
Histologically, the dentate, or pectinate, line marks the separation between the squamous mucosa of the distal anal canal and the transitional epithelium that “transitions” into the glandular mucosa of the distal rectum. As noted, definitions have varied in the literature, but the anal canal can be considered to extend from the anal verge, or orifice, where the perianal skin merges with the squamous cell mucosal lining of the distal anal canal, to the top of the anal sphincter mechanism, where the external anal sphincter muscle joins the puborectalis segment of the levator ani muscle at the anorectal ring. Although variable, the length of the canal typically measures about 4 cm. Some series define the anal canal as the canal between the anorectal ring and the dentate line.1 As opposed to exact location within the canal proximal to the anal verge, histology is generally the more important feature of a tumor with regard to management options. The term “anal margin” is also variably used in the literature, but it is frequently defined as the perianal skin within 5 cm of the anal verge.2 Figure 6.1 is a depiction of the anal canal.
Figure 6.1 Anatomy of the anal canal. Anatomical features of the anal verge, anal canal, and sphincter musculature. (Reproduced with permission from Ryan, D.P. & Willett, C.G. (2011) Classification and epidemiology of anal cancer. In: UpToDate, Basow, D.S. (ed), UpToDate, Waltham, MA. Copyright ©2011 UpToDate, Inc. For more information, visit www.uptodate.com.)

A variety of malignancies can arise within the anal canal. To simplify matters, these can be divided into squamous cell and nonsquamous cell tumors. Squamous cell tumors are the most common presentation. Histological descriptions such as basaloid/cloacogenic (tumors arising in the transitional mucosa of the proximal canal) are not as relevant for practical management and are no longer commonly used in histopathologic descriptions.3 Tumors arising in this area are considered squamous cell tumors and are managed accordingly. Nonsquamous cell tumors including adenocarcinomas, melanomas, lymphomas, and sarcomas have been described, but are less common. A suspected anal adenocarcinoma may in fact be an extension from a distal rectal adenocarcinoma in some scenarios. Ultimately, tumor location within the anal canal is not as important as histological subtype; true anal canal adenocarcinomas are treated as conventional rectal adenocarcinomas, as will be discussed later.
Tumors that lie near, but definitively outside of, the anal verge (perianal skin lesions) are typically squamous cell skin cancers that can be managed as such, although there is controversy regarding this point, which will be addressed later in this chapter. From a practical point of view, invasion locally up to or through the anal verge may impact treatment strategies for these tumors.
Squamous cell anal canal tumors are relatively uncommon, with about 5000 new presentations annually in the United States.4 However, the incidence has climbed steadily in recent years. Epidemiological investigations have identified a number of risk factors associated with the development of squamous cell anal tumors. An increased number of sexual partners have been associated with an increased risk of developing anal cancer.5 In one large case-control series, an increasing number of sexual partners were associated with the development of anal cancer in both men and women (odds ratio of 4.5 for women and 2.5 for men with ≥10 sexual partners).5 This study also demonstrated that a history of anal warts was associated with a higher risk of developing anal cancer, as was receptive anal intercourse in women. Other reports have also shown a relationship between receptive anal intercourse in men who have sex with men and subsequent development of anal cancer.6 Similarly, a prior history of cervical and genital malignancies has also been found to be associated with a higher risk of subsequent development of anal cancer.7,8
These results strongly suggest that a sexually transmitted factor is important in anal cancer carcinogenesis. Thus, it is not surprising that there is a clear association between viral infection with certain subtypes of human papillomavirus (HPV) and development of preinvasive and invasive squamous cell anal cancer, and the majority of squamous cell anal tumors harbor HPV DNA.5,9,10 As with cervical cancer, there are certain subtypes of HPV, in particular HPV-16, commonly found in the cells of malignant lesions. The relationship between high-risk HPV subtype infection and subsequent malignant cellular transformation is well studied in squamous cell carcinomas of the uterine cervix, and as mentioned earlier, women with cervical cancer are also at significantly increased risk of developing squamous cell anal cancer compared with the general population.7 Infection with HPV can lead to a procession from a preinvasive phase (anal intraepithelial neoplasia (AIN), similar to preinvasive cervical lesions) to frank invasive tumors.11
Other identified independent risk factors include non-HIV-related chronic immunosuppression and cigarette smoking.12–14 In another series, patients receiving chronic steroid therapy for amelioration of autoimmunity were also found to be predisposed to HPV-associated anogenital tumor formation. Immunosuppression may prevent effective immune responses to HPV and also hinder antitumor immunity. Cigarette smoking is also associated with development of anal cancer, much as it is with cervical and other cancers.15 The risk of anal cancer appears to be related to the pack-year history of smoking, with more extensive histories associated with a higher risk. The mechanism of the association of smoking with anal tumors is unclear, but there may be a cocarcinogen effect in the context of HPV infection.16
The association with HIV as an independent risk factor for the development of anal cancer is controversial. HIV may not be an independent risk factor for development of anal cancer but may facilitate development of anal cancer in HPV-positive patients17,18 as a result of impaired immunity. In one series, patients who were simultaneously HPV and HIV positive appeared to have a higher risk of developing AIN and anal cancer as opposed to those who were HIV negative. In a separate analysis, patients with low CD4+ T-cell counts were at risk for more aggressive courses of HPV infection.
Diagnosis
Histological confirmation of malignancy by tissue biopsy is required prior to initiation of tumor-directed therapy. Staging, as with most tumors, involves determining local extent of the primary disease, whether or not nodal metastases are present, and whether or not there is evidence for distant spread of disease (outside of the pelvis). Computed tomography (CT) of the chest, abdomen, and pelvis is typically obtained, and digital and endoscopic examination of the anus and rectum, in addition to palpation of the inguinal region, is necessary to delineate the extent of gross tumor.
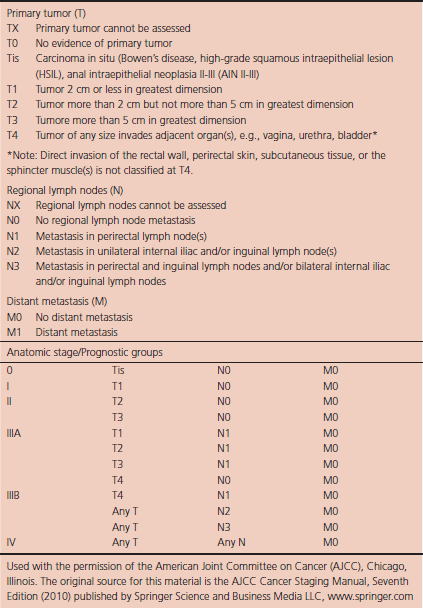
Positron emission tomography (PET) and PET–CT are also now routinely integrated into the staging algorithm for patients.19–21 In one series, PET–CT appeared to have a higher sensitivity than conventional imaging (CT and/or MRI) for detecting regional lymph node metastases (89% vs. 62%), although for practical reasons, not all nodes could be biopsied for a true measure of sensitivity and specificity. PET was found to change planned radiation therapy fields in 13% of patients and thus worthy of inclusion in the staging process.21 Of note, HIV-positive patients may have false-positive fluorodeoxyglucose (FDG)-avid lymph nodes. Biopsy may be necessary in these situations to determine whether or not there is true nodal metastatic disease versus a benign inflammatory process. Table 6.1 describes the current staging system.
Table 6.1 Anal cancer: Staging
Prevention
As mentioned earlier, AIN is thought to represent the precursor lesion to invasive anal cancer. AIN is broadly subdivided into low- and high-grade AIN. Given the success of cervical cancer screening in women, there has been a natural interest in the potential value of screening of patients at high risk for development of AIN and subsequent anal cancer. When performed, screening is usually in the form of analysis of anal cytology obtained by swabbing the anal canal (“anal Pap smear”). Sensitivity for detection of dysplasia appears higher (approximately 75%) in HIV-positive patients as opposed to HIV-negative patients (approximately 60%).22,23 It is also higher in HIV-positive patients with lower CD4 counts.22 In patients with abnormal cytology, anoscopy with administration of 3% acetic acid can then be performed to guide biopsies, much as is done with cervical colposcopy.
Treatment of high-grade AIN, thought to be the direct precursor to invasive anal cancer, can take many forms. Larger mucosal lesions can be ablated with anoscopic-directed electrocautery. Smaller lesions can be treated with topical trichloroacetic acid (TCA), topical 5-fluorouracil (5-FU), or imiquimod.24–26 Topical applications yield lesion control in the range of 60–80%.
To date, however, there are no established guidelines for anal cancer screening in high-risk groups as there are for cervical cancer screening. The main debate regards the cost-effectiveness of screening programs. One important study showed annual screening in HIV-positive men who have sex with men to be of value.27 The cost-effectiveness of screening was deemed comparable with the common practice of using prophylactic antibiotics in HIV-positive patients with very low CD4 counts.
Cancer management
Perianal skin tumors
Tumors that lie definitively distal to the anal verge (perianal skin) may be managed as skin tumors. Treatment options for squamous cell tumors of the perianal skin include local excision with or without adjuvant radiation, or radiation with or without chemotherapy. The treatment approach must take into account expected morbidity. Chapet et al.2 reviewed their experience with 26 patients with tumors of the perianal skin (5 patients also had involvement of the anal canal). Twenty-one tumors were ≤5 cm in diameter. Fourteen patients were treated with definitive radiation (with or without chemotherapy), and 12 patients were treated with radiation after initial local excision. The initial crude local control rate was 61.4% (16/26). After salvage surgical treatment, this increased to 80.8%. Five-year cause-specific survival was 88.3%. Khanfir et al.28 reported similar results in their series of 45 patients. Twenty-nine patients underwent local excision preceding radiation. Treatment fields were variable as a function of patient and tumor features. Five-year local–regional control was 78% and 5-year disease-free survival was 86%.
Balamucki et al.29 recently updated the University of Florida experience with definitive radiotherapy and chemoradiotherapy for squamous cell tumors of the anal margin. Twenty-six patients were treated. Two patients developed local recurrence of disease and two patients had regional lymph node recurrence. Ten-year cause-specific survival was 92%. Of note, two patients with clinically node-negative disease who did not receive prophylactic inguinal nodal irradiation developed groin recurrences.
Anal canal adenocarcinoma
Adenocarcinomas of the anal canal are uncommon and in some cases will represent growth of distal rectal adenocarcinomas into the canal. A study of 82 patients from the Rare Cancer Network registry with a diagnosis of anal adenocarcinoma analyzed outcomes based on treatment approach—radiotherapy plus surgery, chemoradiotherapy, and abdominoperineal resection (APR).30 Tumor and patient features were evenly distributed across the three groups. Local–regional control at 5 years was highest in the APR-treated patients (80% vs. 64% with chemoradiotherapy and 63% with radiation plus surgery), although the differences were not statistically significant. Moreover, 5-year survival was improved in the chemoradiotherapy group (58%) as opposed to the radiotherapy plus surgery (29%) and APR (21%) groups. In multivariate analysis, chemoradiotherapy was found to be a positive independent factor for disease-free and overall survival. A review of 165 patients with anal adenocarcinoma from the Surveillance, Epidemiology and End Results (SEER) database yielded differing conclusions.31 Five-year survival for patients treated with APR was 58%, compared with 50% for APR plus radiation and 30% for radiation alone. These differences were statistically significant. Finally, a report from MD Anderson Cancer Center analyzed 16 patients with anal adenocarcinoma and compared outcomes with chemoradiotherapy treatment with similarly treated patients with squamous cell tumors.32 Five-year rate of local failure was 54% for the adenocarcinoma group and 18% for the squamous cell group, with corresponding 5-year disease-free survival worse for the adenocarcinoma group (19% vs. 77%), as was overall survival (64% vs. 85%).
As a result, patients with anal adenocarcinoma are generally treated as if they had similarly staged rectal adenocarcinoma, with surgery the cornerstone of therapy and (neo) adjuvant chemoradiotherapy reserved for high-risk features (T3 or T4 disease and/or nodal involvement).
Anal canal melanomas
Melanomas of the anorectum are rare, representing about 1% of all anal canal tumors. Prognosis tends to be very poor, with 5-year overall survival rates as low as 6%, usually associated with development of distant metastases.
Appropriate local therapy remains a controversial area, although it appears that obtaining a negative surgical margin may be more important than the actual extent of the surgery. Nilsson reviewed 251 presentations from the Swedish National Cancer Registry, and on multivariate analysis, the two most important prognostic factors with respect to survival were surgical margin status and tumor stage.33 Patients with an R0 surgery had a 5-year survival rate of 19% as opposed to 6% for those with positive margins. Iddings et al.34 reviewed data regarding anorectal melanoma from the SEER database from 1973 to 2003. One hundred forty-three patients were recorded as having localized disease: 51 underwent APR and 92 underwent local excision. Patients between the two groups had “similar” pathologic features, and there were similar outcomes between the two treatments: median survival was 16 and 18 months in the APR and local excision groups, respectively, and 5-year survival was 16.8% and 19.3% for APR and local excision, respectively.
Investigators from MD Anderson Cancer Center reported on 23 patients with anorectal melanomas treated to the primary site with local excision, with or without nodal dissection based on clinical presentation.35 Nine patients received some form of systemic therapy. A dose of 30 Gy was delivered in five fractions to the primary tumor site (to the level of the bottom of the sacroiliac joints) and inguinal nodes. Four patients received an additional 6-Gy boost dose to the primary site. Local and regional nodal control rates at 5 years were 74% and 84%, respectively. Five-year overall survival was 31%, and no patients with regional lymph node involvement at presentation were alive at 5 years.
Anal canal squamous cell cancer
Surgery
Prior to the use of curative-intent chemoradiotherapy, surgery was the mainstay of treatment for squamous cell anal canal tumors. Surgical options include local excision for small and minimally invasive tumors or more radical extirpation such as APR with formation of a permanent colostomy.
Boman et al.36 reviewed the Mayo Clinic experience with 188 patients with anal canal carcinoma. One hundred seventy-two patients had squamous cell or nonkeratinizing basaloid carcinomas (tumors of the proximal anal canal in the transitional zone). Nineteen patients with small tumors confined to the “anal epithelium and subepithelial connective tissue” were treated with local excision only. Only one of these patients had disease failure and was salvaged with APR. Of 118 patients undergoing APR, disease failure was seen in 46 patients. Of these patients, patterns of failure were known in 38. Thirty-two of the 38 patients had a component of local–regional failure. In a separate series of results for anal cancer treatment from Roswell Park, the crude regional recurrence rate following local excision and APR for a variety of stages was 60%.37
As will be discussed in more detail in the following section, concurrent radiation therapy and chemotherapy emerged as a sphincter-sparing alternative to resection. Although there are no randomized trials comparing APR with chemoradiotherapy, local–regional tumor control rates and overall survival with chemoradiotherapy at least rival (if they are not superior to) those obtained with surgery with the added advantage of sphincter preservation. In an overview of the University of Minnesota’s treatment experience with anal cancer, 21 patients with squamous cell anal cancer were treated with surgery, whereas 122 were treated with chemoradiotherapy.38 Nearly half of the chemoradiotherapy-treated patients had T3 or T4 tumors, whereas only 14% of the surgery group had T3 tumors. Despite the more advanced tumors in the chemoradiotherapy cohort, overall 5-year survival was similar between the two groups—60% in those undergoing surgery and 55% in those treated with chemoradiotherapy. Tumor recurrence was found in 23% of the surgical group and 34% of the chemoradiotherapy group.
Concurrent chemotherapy and radiotherapy
Given the relatively poor local–regional control results obtained with surgery alone for locally advanced anal tumors, Nigro et al.39 at Wayne State University instituted a protocol incorporating concurrent pelvic radiation therapy and chemotherapy (5-FU and mitomycin-C (MMC)) as induction therapy prior to surgery for anal canal cancers (defined in their series as tumors at and just proximal to the dentate line). The total radiation dose in the original report ranged from 30 to 35 Gy, and in subsequent series, it was 30 Gy, delivered at 2 Gy per fraction.39,40 When pathologic complete responses were obtained in their original cohort, it became clear that chemoradiotherapy could provide definitive therapy, allowing preservation of the anal sphincter musculature, and APR could be reserved for locally persistent or recurrent disease. Other series using similar chemoradiotherapy treatment protocols also showed high rates of disease response, including pathologic complete response.41,42 Radiation (typically to higher doses in the original Nigro series, at least 45 Gy) and concurrent 5-FU and MMC remain the “standard of care” treatment for most patients with squamous cell anal canal tumors.
Three randomized phase III clinical trials helped to establish this standard.43–46 Two of the studies, the United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Anal Cancer Trial (ACT I) and the European Organization for Research and Treatment of Cancer (EORTC), had similar designs. In the ACT I study, 568 eligible patients with anal canal or anal margin cancer were analyzed.43,44 Randomization was to radiation (45Gy in 20 or 25 fractions, followed by an additional dose to “good responders” of 15 or 25 Gy after a 6-week break) alone or radiation with infusional 5-FU (1000 mg/m2 for 5 days or 750 mg/m2 for 5 days, given during the first and last weeks of radiation) and MMC (12 mg/m2 delivered on day 1 of treatment). Local–regional failure rates were significantly higher in the patients treated with radiation alone (25.3% absolute difference at 12 years), and relapse-free survival was similarly higher in the chemoradiotherapy group (12% absolute difference at 12 years). Cause-specific, but not overall, survival was higher in the chemoradiation group. There was no significant difference between the two groups with respect to late morbidity.
In the EORTC study, 103 eligible patients with T3-4N0-3 or T1-2N1-3 anal cancer were treated with radiation therapy (45 Gy, with a boost dose of 15 or 20 Gy following a 6-week break based on disease response) with or without concurrent chemotherapy (infusional 5-FU at a dose of 750 mg/m2 daily for days 1–5 and 29–33, with MMC also given on day 1 at 15 mg/m2).45 Patients treated with chemotherapy had a higher complete response rate than those treated with radiation alone: 80% versus 54%. Local–regional control, colostomy-free survival, and event-free survival were all higher in the chemoradiotherapy group at 5 years. Rates of high-grade toxicity were similar between the two groups, although rates of late anal ulceration were higher in the combined modality group. Overall survival was superior in the chemoradiotherapy treatment arm, but this did not reach statistical significance.
A third study, conducted by the Radiation Therapy Oncology Group (RTOG) and the Eastern Cooperative Oncology Group (ECOG), attempted to de-intensify therapy by omitting MMC, hoping to maintain oncologic efficacy while eliminating the toxicities associated with MMC.46 Patients with anal canal cancer of any T- or N-stage were randomized to radiation therapy and 5-FU plus MMC versus radiation and 5-FU alone. The radiation dose was 45–50.4 Gy depending on treatment response, with an additional 9 Gy delivered to patients with biopsy-proven persistent disease 4–6 weeks following completion of initial treatment, and an additional 9 Gy delivered to residual palpable inguinal lymph nodes. Infusional 5-FU was delivered at 1000 mg/m2/day on days 1–4 and 29–32. MMC was delivered at 10 mg/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








