and Armanda Tatsas2
(1)
Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
(2)
Pathology Group of Louisiana, Baton Rouge, LA, USA
Introduction
The majority of adrenal lesions, masses, or cysts are incidentally detected during imaging studies performed for investigation of extra-adrenal diseases. Most of these “incidentilomas” are benign, nodular hyperplasias, or adenomas, but other nonfunctional cortical or medullary neoplasms, even rarely, metastatic neoplasms without known primary malignancies, can be detected. In most cases, the decision for the management of these patients (type of treatment or follow-up without treatment) can be made based on the size and other imaging characteristics. Small masses (<3 cm) are usually benign, and larger ones (>4 cm) are malignant, but small malignant and large benign neoplasms have been reported.
Fine Needle Aspiration (FNA)
The use of FNA in the diagnosis of adrenal lesions is usually limited to single adrenal masses in patients with extra-adrenal malignancy to determine the nature of the mass. Most FNAs are performed by ultrasound (US) or computed tomography (CT) guidance. Endoscopic ultrasound (EUS) guided FNAs, almost all done on the left adrenal gland, have also been used in recent years. On-site evaluation (OSE) of the specimen helps to obtain adequate samples and direct the material for ancillary studies as needed (see Chap. 2). Complications of FNA include hematoma in the adrenal gland and pneumothorax. A few cases of tumor seeding in the needle tract have been reported.
Cysts
Benign cysts of adrenal gland are rare. They can be pseudocysts, vascular or epithelial. Aspiration material contains macrophages, leukocytes, and benign epithelial cells as well as erythrocytes and hemosiderin-laden macrophages in hemorrhagic cysts.
Nodular Cortical Hyperplasia and Cortical Adenoma
Most of the incidentally detected adrenal masses represent hyperplastic nodules or cortical adenomas. They can be functional or nonfunctional. Cytomorphology of hyperplastic nodules and adenomas, functional or nonfunctional, is similar and is presented together.
Cytomorphology:
Specimens are usually cellular and composed of uniform single cells, aggregates, and tissue fragments in varying proportions. Individual cells have round nuclei with smooth borders and finely vacuolated cytoplasm (Fig. 6.1). Scattered large cells with larger nuclei may be present (Fig. 6.2). Because of the fragile cytoplasm, bare nuclei in a “bubbly” background are commonly seen. In cellular specimens, aggregates of bare nuclei could be mistaken as small cell carcinoma (Fig. 6.3).
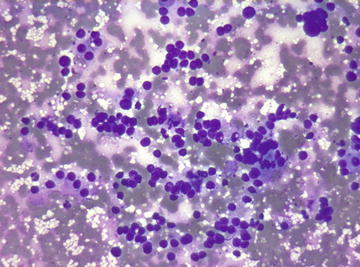
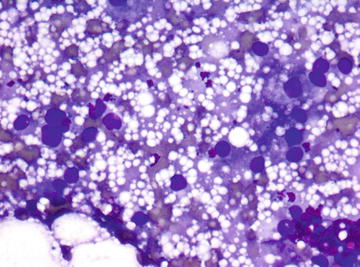
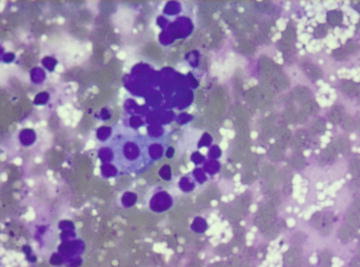

Fig. 6.1.
Adrenal cortical adenoma: Groups and single cells with round nuclei in a background of lipid vacuoles. Most cells have lost their cytoplasm or have ill-defined cytoplasmic borders. (Diff-Quik stain, medium power)

Fig. 6.2.
Adrenal cortical adenoma: Several cells with large nuclei. (Diff-Quik stain, high power)

Fig. 6.3.
Adrenal cortical adenoma: Several tight groups of bare nuclei mimicking small cell carcinoma. (Diff-Quik stain, high power)
Key features:
Cells with round uniform nuclei and finely vacuolated cytoplasm
Bare nuclei in a background of lipid droplets
Differential diagnosis:
Benign proliferations of cortical cells, including adenomas, cannot be differentiated from adrenocortical carcinoma in FNA or core biopsy specimens. Metastatic tumors with clear cell features, specifically renal cell carcinomas and some hepatocellular carcinomas, may have similar morphologic features to those of adrenocortical neoplasms. Immunohistochemical profile of these tumors helps to differentiate them from adrenal cortical neoplasms, which will be discussed under adrenal cortical carcinoma.
Myelolipoma
Myelolipoma is a rare benign tumor of adrenal gland usually found incidentally during abdominal imaging studies. They can reach large sizes. FNA provides a definitive diagnosis.
Cytomorphology:
Characteristic findings are a mixture of fat tissue and hematopoietic cells. Erythroid, myeloid precursors, and megakaryocytes mixed with fat tissue or fat droplets are typically found (Fig. 6.4).
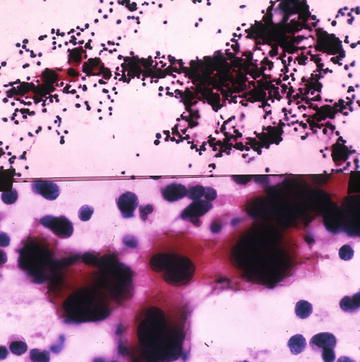

Fig. 6.4.
Myelolipoma: Top, mixture of adipose tissue and myeloid and lymphoid elements. (Papanicolaou stain, low power) Bottom, megakaryocytes. (Papanicolaou stain, high power). (Top) Published with permission from: Fine Needle Aspiration Cytology, eds. MK Sidawy, SZ Ali, Churchill Livingston/Elsevier, 2007, Chapter 10, Kidney and Adrenal Glands, YS Erozan, pages 299–346
Key feature:
Hematopoietic cells admixed with fat
Differential diagnosis:
In adequate samples, the cytopathological findings are quite specific. Extramedullary hematopoiesis may be considered in the absence of fat tissue. The latter usually occurs at multiple sites and is associated with other conditions causing failure of hematopoiesis in bone marrow.
Pheochromocytoma
Pheochromocytoma is a rare neoplasm of adrenal gland arising from the chromaffin cells of the medulla. Over 90 % of the cases are sporadic. Familial cases belong to one of the multiple endocrine neoplasia (MEN) syndromes. Sporadic cases in adults are usually solitary masses.
The majority of pheochromocytomas are functional. Increased levels of vanillylmandelic acid (VMA) and catecholamines are found in the urine of about 90 % of patients with these tumors and are used in the diagnosis along with imaging studies. About 10 % of these tumors are malignant. In most publications, suspicion of pheochromocytoma is considered a contraindication for FNA because of the potential risk for hypertensive crisis and unstoppable bleeding. These complications, however, are rare; and FNA is performed in some cases, even if the possibility of pheochromocytoma exists. In these cases, it is recommended that the radiologist be prepared to deal with those complications.
Cytomorphology:
FNA general provides hypercellular specimens composed of single cells and loose cell groups. The tumor cells have varying morphology. Large pleomorphic cells, smaller neuroendocrine type cells, and spindle cells have been described. Binucleated and multinucleated cells and occasional intranuclear pseudoinclusions are present (Fig. 6.5a, b).
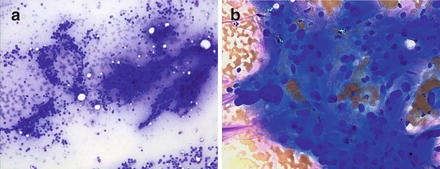

Fig. 6.5.
Pheochromocytoma: (a) Single cells and tissue fragments with uniform round or ovoid nuclei with ill-defined cytoplasm. Several rosette formations are present. (Diff-Quik stain, low power) (b) Syncytial appearing tissue fragment and single cells. Markedly pleomorphic nuclei. (Diff-Quik stain, high power)
Immunohistochemistry:
Stains for NSE, synaptophysin, and chromogranin are positive (Fig. 6.6). S-100 stains sustantecular cells.
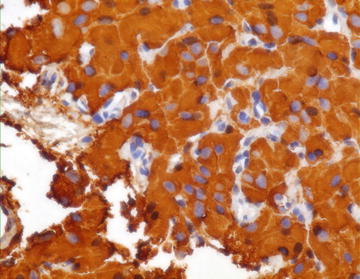

Fig. 6.6.
Pheochromocytoma: Strong positivity with Chromogranin stain. (Cell block, medium power)
Key features:
Cellular specimens with single cells and loose groups of cells
Large pleomorphic cells and smaller neuroendocrine-type cells
Binucleated and multinucleated cells
Differential diagnosis:
The main differential diagnoses include adrenocortical carcinoma, sarcomatoid renal cell carcinoma and, rarely, retroperitoneal sarcoma. Immunohistochemical stains are usually needed for differentiating pheochromocytoma from the others (Table 6.1).
Table 6.1.
Immunohistochemistry of neoplasms metastatic to adrenal glands.
Neoplasms | Positive immunostains |
|---|---|
Lung | |
Adenocarcinoma | TTF1, Napsin A |
Small cell carcinoma | TTF1 |
Breast | ER, Mammoglobin, GCDFP, GATA3 |
Colon | CK20, CDX2 |
Stomach | CK20, CK7 |
Pancreas | CK 19-9 |
Endometrium | ER, PR |
Ovary, serous
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




