The prescription of chronic peritoneal dialysis involves a number of elements. Initially, there is the choice of peritoneal dialysis modality between continuous ambulatory peritoneal dialysis (CAPD) and cycler or automated peritoneal dialysis (APD) and their variants. Then there is the selection of a specific prescription based on clearance, ultrafiltration, and nutritional/metabolic requirements. The term “adequacy” is often used in this context and usually refers specifically to the quantity of clearance delivered but can also be used in a broader sense to reflect the quality of the dialysis prescription as a whole. Please review
Chapter 21 (Physiology) and 22 (Equipment) at this time, as many concepts discussed in those chapters will not be repeated here.
A. Modalities of peritoneal dialysis therapy
1. CAPD. The simplicity of CAPD, the ease of doing it at home, its relatively low cost, and the associated freedom from dialysis machinery have combined to make it historically the most popular chronic peritoneal dialysis modality. It provides continuous therapy and a steady physiologic state. Control of body fluid volume can usually be achieved, and normalization of blood pressure is possible in most patients.
The principal disadvantage of CAPD for many patients is the requirement for multiple procedural sessions (usually four per day), each taking up 30-40 minutes of patient time. While these can be done away from home, the requirement for sterility and access to supplies usually means that the patient returns home, and so this may constrain daily activities somewhat. Frequency of procedures may also be an issue where relatives or other caregivers are carrying out the exchanges for the patient. Other factors are limitations on dwell volumes due to increased intraperitoneal pressure and a limited range of solute clearance. Episodes of peritonitis occurring as often as once every 12 months were a significant disadvantage in the past; however, with improved
transfer sets and connecting devices, such occurrences have been markedly reduced and successful programs report rates of one peritonitis episode every 3 years or fewer.
2. APD. This has become very popular over the past 10-15 years and, in many wealthier countries, is being used in the majority of peritoneal dialysis patients. The main advantage of APD, compared with CAPD, is the lesser number of on-off procedures required each day—typically two versus four for CAPD—and none during the daytime. All connections and preparation of equipment usually take place in the privacy of the home so that psychological adjustment is facilitated and patient fatigue and “burnout” may be reduced. APD is an attractive treatment option for active individuals who would be inconvenienced by the interruptions in daily routine that are required with CAPD. APD is also the therapy of choice for most patients who require assistance in carrying out their dialysis (e.g., children, the dependent elderly, and nursing home residents).
The main disadvantages of APD relative to CAPD are the need for a cycler, the greater cost, and the slightly greater complexity.
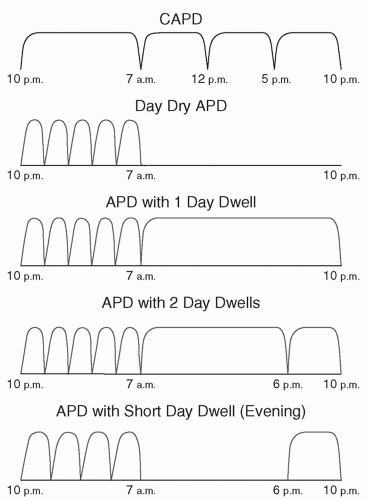
APD has classically been divided into APD with a day dwell, often called continuous cycling peritoneal dialysis (CCPD), and day dry APD (
Fig. 25.1), often called nocturnal intermittent peritoneal dialysis (NIPD). These modalities have already been described in
Chapters 21 and
22.
An alternative form of APD is tidal peritoneal dialysis (TPD). This modality uses an initial fill volume followed by partial drainage at periodic intervals (
Fernando, 2006). The principal purpose of TPD was to enhance clearance of small solutes by avoiding the normal loss of dialysis time associated with inflow and drainage. In terms of clearance, the advantage of TPD over standard APD is not seen unless very large quantities of dialysis solution are used. The main use of TPD nowadays is to minimize drain pain during nighttime cycling. The main disadvantage of high-volume TPD is increased cost and complexity, and it is not widely used.
B. CAPD or APD: Which modality to choose? This decision should take into consideration both patient preferences and the need to provide a medically optimal peritoneal dialysis prescription. Patient preferences may be based on lifestyle, employment, place of residence, ability to perform the various modalities of PD, comfort with cycler technology, and the degree of family and social support. In the past, peritoneal transport status and its influence on clearance and fluid removal were thought to be critical in choosing between CAPD and the different types of APD, but there is now an increasing sense that these aspects were overstated and that lifestyle factors should be given more emphasis.
APD was previously thought to be better than CAPD for managing volume status. However, the phenomenon of
sodium sieving (see
Chapter 26) is more apparent with the short-cycled dwell times of APD, and this, along with the risk of net fluid resorption with long day dwells, has led to concerns about adequacy of sodium removal with APD. One recent study suggests less salt removal and a higher prevalence of systolic hypertension with APD than with CAPD, but this was not a randomized trial, and there is no consensus that these findings are generalizable (
Rodriguez-Carmona, 2004). Salt and water removal require close attention on both CAPD and APD, but there is insufficient evidence to justify it being a factor in initial modality selection.
Risk of peritonitis is another medical factor that may arise when deciding between CAPD and the variants of APD. One randomized trial done over two decades ago showed less peritonitis on APD, but both modalities have changed since then, and there is now no consensus that one or the other is more likely to predispose to peritonitis.
A third consideration is cost. CAPD generally is cheaper than APD. Dialysis programs have to deal with financial constraints, and in some settings, patients may have to bear some or all of the costs.
II. CHOICE OF A PrEsCrIPtIOn
A. Clearance targets
1.
Weekly Kt/V urea. Clearance targets in PD are set in terms of weekly urea clearance (
Kt) normalized to the patient’s estimated urea distribution volume (
V). Current guidelines aim for a
Kt/V urea target of at least 1.7. Previously, this target had been set higher, at 2.0 or even greater for noncontinuous forms of PD, but the guidelines were lowered based on further trial evidence, and in particular, the randomized ADEMEX study (
Paniagua, 2002), which found no difference in outcomes between patients assigned to receive a higher versus a lower dose of PD. In the ADEMEX trial, the average weekly
Kt/V was 2.1 in the patients assigned to more dialysis, compared to 1.6 in the lower-dose group. Current guidelines do not set different targets for continuous and noncontinuous forms of PD (e.g., day dry APD), nor do they set different targets based on peritoneal transport status. A similar trial from Hong Kong (
Lo, 2003) also failed to find a benefit of higher doses of PD.
2.
Weekly creatinine clearance (CrCl) per 1.73 m2. Previous guidelines also set a weekly CrCl target in addition to the
Kt/V urea target. The creatinine target was normalized to 1.73 m
2 body surface area and was in the range of 60/1.73 m
2 L per week. The idea of setting a separate creatinine target was to model a uremic toxin that had slightly higher molecular weight than urea (113 vs. 60 Da) and that was not so rapidly removed by diffusion. Most current guidelines no longer recommend a minimum level of weekly CrCl as such targets have not been shown to be of any additional value over
Kt/V targets. However, they do reflect clearance of slightly larger molecules than urea, and so European, but not US, guidelines suggest an additional CrCl target of 45/1.73 m
2 L per week (
Dombros, 2005).
3.
should residual kidney function be counted in the adequacy target? Greater residual renal clearance has repeatedly been shown to be associated with superior patient survival; in fact, it has been difficult to show a similar survival effect for peritoneal clearance, at least within the range of prescriptions in typical clinical use (
Churchill, 1995). Some have suggested that the weekly
Kt/V urea target of 1.7 should be met by peritoneal clearance alone and that residual renal clearance should be treated as a precious bonus. KDOQI, Canadian, and European guidelines, however, all recommend that peritoneal and renal
Kt/V can be added to achieve the target.
4. same Kt/V target for CAPD and APD. The previous idea that target clearances for APD should be higher than those for CAPD because APD is somewhat more intermittent is now thought to be unjustified and to introduce unnecessary complexity.
B.
Measurement of clearance (
Table 25.2). Clearance in peritoneal dialysis can be measured in terms of
Kt/V urea and additionally as CrCl/1.73 m
2. Both clearances comprise a peritoneal and a residual kidney component. Residual kidney function lasts longer in PD than in hemodialysis and accounts for a greater proportion of total clearance.
1.
Measurement of weekly Kt/V urea. Peritoneal
Kt/V is calculated by performance of a 24-hour collection of dialysate effluent and measurement of its urea content. This is then divided by the average plasma urea level for the same 24-hour period to give a clearance term,
Kt (
Table 25.3). The timing of the plasma urea sample is not critical in CAPD because it is relatively constant at all times. In APD, blood urea is not quite so constant throughout the day; ideally, therefore, it is best to take a measurement in the middle of the noncycling daytime period, which is typically between 1:00 p.m. and
5:00 p.m. and is thought to represent approximately the average blood urea levels for the day.