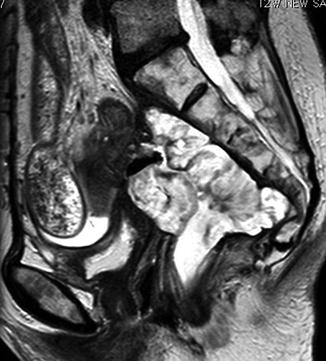
Fig. 10.1
T2 weighted MRI showing invasion of tumor posteriorly into the sacrum. The arrow shows tumor invasion within the posterior compartment
Despite all the recent developments in surgery and medical therapy, up to 40 % of the patients that undergo surgery for primary rectal cancer will develop local recurrence and/or distant metastases [1–4]. Local recurrence rates have been reported as low as 2.5 % [5]. They can range up to 30 % though, with distant hepatic or lung metastases diagnosed in up to 20 and 9 % of patients respectively [4]. The majority of the recurrences will be diagnosed within the first 3 years following surgery [6]. A third of these patients will be free of distant metastases and may be eligible for a curative resection. For this group of patients, radical resection is the only option for cure, as chemotherapy and radiotherapy are unlikely to be curative and are used for palliation when used alone. Curative resection is feasible in less than a third of cases [7].
Rectal cancer surgery was initially performed during the eighteenth century with the first two reported resections resulting in the patients dying [8]. LisFranc was the first to perform a “successful” oncological resection of the rectum [9]. Perioperative morbidity was high though and was associated with poor disease free and overall survival. All operated patients that survived the operation represented with a recurrence and died.
It was the introduction of anesthesia and aseptic technique that enabled the improvement of the surgeons’ performance that consequently resulted in the improvement of the perioperative outcomes. The first anatomical resection was performed by Ernest Miles [10]. He removed the draining lymph nodes while resecting the rectum, by combining the abdominal and perineal approach improving the oncological outcomes [11]. However, the functional outcomes and quality of life was adversely affected due to the presence of a colostomy and the poor sexual and urinary function.
Rectal cancer surgery was revolutionized in the late twentieth century when Professor Heald introduced the total mesorectal excision [12]. This was based on the embryologic development of the hindcut, after studies demonstrated that resection margins of 2 cm should be considered as safe. This had led him to further study the value of “holy plane” and proposed a standardized oncological rectal surgery by performing total mesorectal excision of the rectum [13].
The Japanese surgeons recommended the excision of the lateral pelvic sidewall lymph nodes to supplement the standard operation. The results from a number of studies were controversial though with a meta-analysis showing that the benefit from an extended lymphadenectomy did not seem to offer a significant oncological advantage while at the same time was shown to be associated poor sexual and urinary function [14].
The introduction of neo-adjuvant radiotherapy to the management of rectal cancer signified the reach for another important milestone. Its role was established late in the twentieth century when studies demonstrated significant reduction of recurrence rates but without any significant impact on the patients’ long term survival.
The development and evolution of all the above techniques along with the acquired knowledge from the “mistakes of the past”, have resulted in the progressive reduction of the local recurrence rates. However, the recurrence rates are still considered high, necessitating radical surgery to completely remove the cancer.
There is a significant variation in the patterns of recurrence and therefore the management plan should be titrated to the individual. A surgical plan can be made with the help of imaging modalities such as MRI and CT scan. The images from these modalities have been significantly improved in the recent years allowing better detection rates and identification of earlier recurrences. This has subsequently facilitated the performance of more operations for this group of patients.
Isolated anastomotic recurrences can be amenable to local resection but more extensive disease requires a more radical resection. Pelvic exenteration when the tumor invades adjacent structures, with lateral extended lymphadenectomy when there is invasion into the lateral pelvic structures. When the tumor invades the sacrum, removal of the tumor along with the sacrum is required.
The absence of accurate diagnostic tools, lack of knowledge and the anatomical/surgical challenges, have resulted in a delay in the attempts to resect recurrent rectal tumors. It was not until the mid-nineties that advanced pelvic exenterative surgery for locally advanced primary and recurrent colorectal cancer was considered as an option for cure. The first pelvic exenterative procedures were described in 1948 [15] and were associated with high mortality and morbidity rates. Numerous studies have been published since (Table 10.1) with a significant variation in the mortality and morbidity rates. This is primarily attributed to the differences in the patient selection among the studies’ population. In 1981, Wanebo [32] was the first to report on the outcomes following abdominosacral resection in 11 patients with locally advanced primary (1 patient) and recurrent colorectal pelvic cancer 10 patients. All patients had neo-adjuvant radiotherapy. Plastic reconstruction surgery was performed to close the pelvic and perineal defect. The reported mortality and morbidity rates were also high.
Table 10.1
Recent Studies reporting on abdominosacral resection for locally advanced primary and recurrent rectal cancer
Author | Year | Curative intent | R0 (n) | R0 (%) | R1 (n) | R1 (%) | R2 (n) | R2 (%) | Mortality (%) | Morbidity (%) | 5 years Survivawl (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
Bosman [16] | 2014 | 86 | 48 | 55.81 | 30 | 34.88 | 8 | 9.30 | 3.5 | – | 28 |
Bhangu [17] | 2012 | 30 | 23 | 76.67 | 7 | 23.33 | 0 | 0 | 0 | 50 | |
Sagar [18] | 2009 | 40 | 20 | 50 | 19 | 47.5 | 1 | 2.5 | 2.5 | 60 | – |
Ferenschild [19] | 2009 | 25 | 19 | 76 | 4 | 16 | 2 | 8 | 0 | 68 | 30 |
Williams [20]a | 2008 | 3 | 3 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | – |
Akasu [21] | 2007 | 44 | 24 | 54.54 | 13 | 29.55 | 7 | 15.90 | 2 | 61 | 34 |
Melton [22] | 2006 | 29 | 18 | 62.07 | 10 | 34.48 | 1 | 3.44 | 3.4 | 59 | 43 |
Moriya [23] | 2004 | 57 | 48 | 84.21 | 9 | 15.79 | 0 | 0 | 3.5 | 58 | 42 |
Gonzalez [24] | 2003 | 45 | 33 | 73.33 | 12 | 26.67 | 0 | 0 | 4 | 56 | 32 |
Yamada [25] | 2002 | 64 | 51 | 79.69 | 13 | 20.31 | 0 | 0 | 1.6 | 56 | – |
Mannaerts [26] | 2001 | 50 | 26 | 52 | 18 | 36 | 6 | 12 | 0 | 82 | – |
Weber [27] | 2000 | 23 | 21 | 91.30 | 2b | 8.70 | 0 | 0 | 0 | 43 | – |
Zacherl [28] | 1999 | 12 | 12 | 100 | 0 | 0 | 0 | 0 | 0 | 42 | – |
Wanebo [29] | 1999 | 53 | 45 | 84.91 | 8b | 15.09 | 0 | 0 | 8 | – | 31 |
Magrini [30] | 1996 | 16 | 14 | 87.5 | 2 | 12.5 | 0 | 0 | 0 | 50 | – |
Wanebo [31] | 1994 | 47 | 40 | 85.11 | 7b | 14.89 | 0 | 0 | 8.5 | – | 33 |
The development of the technology and the knowledge acquired from performing these procedures had led to the improvement of the patient selection, surgical technique and medical therapy. Studies from various tertiary centers in the world have been recently published showing an improvement of the oncological results with the mortality and morbidity rates to remain at high levels though.
The focus of this book chapter is the technique of the abdominosacral resection and this will be discussed more extensively within the book chapter.
Patterns of Recurrence
Tumor recurrence may invade any of the intrapelvic structures. With the absence of the mesorectum, the adjacent organs and structures are “unprotected” and susceptible to the tumor. The tumor may be progressing anteriorly, posteriorly, laterally or inferiorly. In some occasions it may be isolated at the anastomosis or invading the peritoneum and/or large and small bowel. Published data suggest that the posterior intrapelvic compartment that includes the presacral fascia, retrosacral space and sacrum, is the most common site of local recurrence, representing up to 56.6 % of local recurrences [29, 33–36]. Invasion within this compartment necessitates the performance of an abdominosacral resection to completely remove the tumor. In the majority of the cases tumor will be invading multiple compartments requiring multi-compartmental resection to remove the tumor en bloc.
The Anterior below the peritoneal reflection compartment has been shown to be the second most common site of recurrence, ranging up to 50.9 % of local recurrence [29]. This includes the genitourinary system. Invasion of the lateral compartment structures has been demonstrated to be up to 26.7 % of local recurrence [34]. Lateral compartment structures include the ureters, iliac vessels, lateral pelvic sidewall lymph nodes, fascia and bone as well as the roots of the sciatic nerve. This compartment in in continuity with the posterior compartment and often can be affected by the tumor. Tumor invasion within this compartment increases the risk of an incomplete resection. Anastomotic (central compartment) recurrence has been shown to range up to 33.9 % of local recurrences [33, 37–39]. Involvement of the perineum and perineal scar (inferior compartment) following abdominoperineal excision of rectum (APER) has been shown to be up to 14 % of all recurrences [40]. Extent of tumor within a bowel loop has been also reported at the range of 14 % [40].
Surgery for Locally Advanced Primary and Recurrent Rectal Cancer
The introduction of total mesorectal excision has significantly improved the local recurrence rates and patients survival. However, local recurrence rates are still considered to be relatively high with significant variation between centers and among surgeons [5, 41–44]. The only potential cure for this group of patients is radical surgery. This can be in the form of exenterative pelvic surgery, including total pelvic exenteration and abdominosacral resection. Patients with locally advanced primary rectal cancer requiring surgery beyond the boundaries of TME, require similar aggressive approach by performing exenterative pelvic surgery and abdominosacral resection when the tumor invades the sacrum.
Abdominosacral Resection
Abdominosacral resection of the rectum was originally performed to surgically treat primary rectal cancers [45–49]. Surgeons were fond of the technique as it provided good exposure of the rectum and tumor, facilitating its wide resection with safe surgical margins and the performance of anastomosis. This procedure was used without the disruption of the anal sphincters and their innervation. One center has suggested the use of abdominosacral resection for the surgical management of low rectal cancers, demonstrating similar oncological and functional results to the patients treated with conventional abdominoperineal excision of the rectum [50–52]. The high morbidity rates and the introduction of total mesorectal excision had led the surgeons to abandon it as treatment for non-advanced primary rectal cancer. Wanebo [32] was the first to perform abdominosacral resection for patients with locally advanced primary and recurrence colorectal pelvic cancers. The results of operating on the first 11 patients were published in 1981, showing high rates of mortality and morbidity.
In the modern era of colorectal surgery, abdominosacral resection is used to treat locally advanced primary and recurrent rectal cancer when the cancer progresses posteriorly and breaches the retro-sacral fascia, therefore potentially threatening the posterior margins. Numerous studies (Table 10.1) have reported their results of performing abdominosacral resections in patients with locally advanced primary and/or recurrent rectal cancer. The colorectal group of Mayo clinic [30] used intraoperative radiotherapy to supplement the abdominosacral resection and reported high rates of complete resections (R0 = 87 %) associated with morbidity (50 %) and poor survival. The results of another published series of 12 patients (all with complete tumor clearance (R0)) [28] that underwent abdominosacral resection for recurrent rectal cancer showed the lowest morbidity at 42 % but 3 year survival as low as 17 %.
Results from studies that followed were more promising though. A study [26] where composite abdominosacral resection was performed in patients with locally advanced primary (n = 13) and locally recurrent rectal cancer (n = 37), demonstrated a 61 % overall local control and 41 % disease free 3 year survival. Yamada et al. [25] used the abdominosacral approach to treat patients with locally advanced primary (n = 15/22; 68.18 %) and recurrent (n = 21/42; 50 %) rectal cancer achieving overall curative resection of 79.69 and 23 % overall 5 year survival. The Memorial Sloan Kettering Cancer Group demonstrated in a series of 29 patients a 62 % complete tumor resection and 20 % 5 year survival with significant morbidity though at the range of 59 % [22]. Moriya et al. [23] reported similar complete clearance rates at 84 %. The Royal Marsden group published the results of 30 patients that underwent abdominosacral resection for locally advanced primary (8 patients) and recurrent rectal cancer (22 patients) showing an overall 66 % 3 year local recurrence free survival, concluding that the procedure is associated with a low margin-positive rate and should be considered as an acceptable treatment for this group of patients [17]. Margin-positive resection was shown to be associated with poor survival outcomes and should be avoided when possible [17]. More studies have been recently performed and reported similar results [18, 19, 21]. One recent study has investigated the feasibility of laparoscopic abdominosacral resection in three patients with locally advanced primary rectal cancer and demonstrated that it is feasible providing an acceptable cosmetic result without compromising the oncological outcome (all patients had a complete tumor resection) [20]. This study reported on only three patients.
Local Staging of Primary and Recurrent Rectal Cancer
Accurate local staging is vital for the management of this group of patients. It can provide information about the local extent of the disease and subsequently the type of surgery that is required to achieve complete removal of the tumor along with the risk of incomplete tumor resection. It also allows a detailed discussion within a multidisciplinary team to plan neo-adjuvant, adjuvant and palliative therapy.
Endorectal Ultrasound
Endorectal ultrasound (EUS) has been used to diagnose recurrent disease with adequate sensitivity and specificity [53, 54]. It is a useful tool as it allows the performance of biopsies at the same time with the procedure. A meta-analysis demonstrated that EUS is very accurate in staging advanced (T4) rectal cancer with 95 % sensitivity and 98 % specificity [55]. However, it provides limited information on the extent of the disease within the adjacent structures and cannot provide adequate information to safely evaluate the tumor resectability. USS has limited field of view and cannot be performed when there is significant stenosis caused by intra-luminal tumor or extra-luminal pressure by tumor [56]. It has limited value following APER when it can only be used transvaginally in female patients to assess tumor invasion in the anterior structures. Therefore its use in the preoperative assessment and staging of this group of patients has been gradually abandoned.
Computed Tomography (CT)
Computer tomography (CT) is the most commonly used radiological modality for detecting primary and recurrent rectal cancer. CT has been demonstrated to have a sensitivity up to 95 % in detecting local recurrence [57, 58]. However, it may often have difficulties differentiating between tissue fibrosis and local recurrence [59, 60]. It has the tendency to overstage bladder involvement [61]. Its accuracy further drops if radiotherapy had previously been applied or in cases that there was previous pelvic sepsis [62]. Its sensitivity is considered low in diagnosing tumor invasion within the anterior structures (bladder and uterus; 50 %) and loco-regional lymph nodes (33 %) [63]. One study assessed the ability of CT scan to determine the extent of the pelvic disease, demonstrating an overall accuracy of 87 % (77.5–93 %) [61]. CT scan for tumors confined in the pelvis was more accurate (89 %) than when tumors were progressing into the abdomen (80 %) [61].
Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging has been demonstrated to be highly accurate in the pre-operative staging of locally advanced primary and recurrent colorectal intrapelvic cancer, providing significant anatomical details that enable the planning of neoadjuvant therapy and surgery [64, 65]. It is now considered the gold standard to stage patients that are considered to undergo exenterative pelvic surgery for locally advanced primary and recurrent rectal cancer. MRI has a fundamental role when surgery is considered as an option for treatment as it accurately depicts the pelvic anatomical structures and compartments relevant to surgery [64, 65]. Previous studies had shown MRI to be highly accurate in detecting colorectal tumor invasion into pelvic structures such as the prostate, seminal vesicles and the sacrum [66, 67]. One more study reported that MRI is accurate in predicting the absence of disease to non-resected organs/structures [68]. Messiou et al. [66] demonstrated that the MRI was highly accurate in diagnosing tumor invasion into individual adjacent to the rectum anatomical structures but proved to be problematic when assessing the pelvic sidewalls (sensitivity = 70 %) and the female reproductive organs (specificity = 33 %). A more recent study demonstrated that it is accurate in predicting the extent of the tumor within the pelvis and can be safely used to guide surgery for curative resection [69]. The same study showed that the MRI sensitivity was very high for all compartments but the specificity was lower for the posterior compartment. Compared to CT, MRI can more accurately differentiate recurrent cancer within a presacral scar, based on differences in signal intensity between tumor and fibrosis using T2-weighted sequences or contrast-enhanced imaging techniques [70].
Diffusion Weighted Magnetic Resonance Imaging (DW-MRI)
Diffusion-weighted MR imaging (Fig. 10.2) is a functional radiological modality that can provide indirect information about the water proton mobility within biologic tissue [71, 72], without the need of a contrast agent [73–75]. As a result, a number of studies have been performed aiming to exploit the features of diffusion-weighted imaging and try to characterize the cellular composition of different tissues. Diffusion weighted MR imaging has since found widespread application in the management of acute cerebral ischemia as it has been demonstrated to be highly accurate in its early detection [76–78].
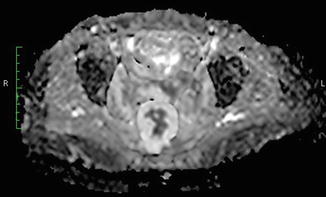

Fig. 10.2
Diffusion weighted MRI showing a cancer recurrence with mucinous component
As a consequence, there has been a rising interest about the diagnostic value of diffusion weighted MRI in oncology. Findings of recent studies suggested that the management of patients with different cancers could be benefitted from the additional information DW-MRI can provide [79–84]. In colorectal cancer, there have been a number of studies investigating the DW-MRI’s efficacy in the management and prediction of oncological outcomes. In a series of 33 patients Ichikawa et al. [85] showed that DW-MRI is highly accurate in detecting colorectal cancer. Sun et al. [86] investigated its value in a series of 37 patients with locally advanced primary rectal cancer, concluding that it can be used to predict tumor response to chemoradiotherapy. Another study compared it with Positron Emission Tomography (PET) in a series of twenty five patients with colorectal cancer and reported it to be inferior in the detection of primary lesions but superior to PET in the detection of lymph nodes metastases. Lambregts et al. [87] however, demonstrated that it is not reliable to stage local lymph nodes following radiotherapy if used alone. The main benefit of adding DW-MRI in the same study was the increased number of detected lymph nodes and the improved positive predictive value for the identification of metastatic lymph nodes. Kim et al. [88] demonstrated that there is a role for DW-MRI as it can improve the diagnostic accuracy of MRI in the evaluation of the tumors’ response to neoadjuvant chemoradiotherapy.
PET and PET/CT
Positron emission tomography (PET) scan is an accurate diagnostic tool and may have advantages over CT and MRI in discriminating fibrosis from cancer [89]. Exploiting the enhanced uptake of FDP-glucose by tumor cells, PET is able to detect both local recurrence and distant metastases. A meta-analysis demonstrated a PET sensitivity and specificity of 94 % for detecting local recurrences [90] with high accuracy in detecting pelvic recurrence in patients who had previously been irradiated [91]. However, limitations of PET scan include the inability to identify small volume disease and a relatively low sensitivity for detecting lymph node metastases [92]. Mucinous adenocarcinomas have poorer FDG uptake and therefore can be easily missed by PET scan [93]. In an effort to increase the confidence in diagnosing recurrence, PET with CT (PET/CT) image fusion was performed. Sapir et al. investigated the role of PET/CT in 62 patients demonstrated that PET/CT was more accurate than PEt alone for detecting local recurrence [94] but is not very helpful in evaluating anatomical tumor changes following chemoradiotherapy [95]. It might be useful in predicting pathological tumor response though [95–97].
Summary of Strengths and Weaknesses of CT, MRI and PET
CT and MRI have demonstrated high sensitivity in detecting local and distant recurrence and can provide detailed anatomical information of the affected organ and tumor extension into surrounding tissues [61, 98]. However, CT may often have difficulties determining if a suspected pelvic mass represents disease recurrence or tissue fibrosis. This becomes even more difficult if radiotherapy had previously been applied or there was previous pelvic sepsis from an anastomotic dehiscence [62].
PET scan is an accurate diagnostic tool and may have advantages over CT and MRI in differentiating scar tissue from cancer [89]. Exploiting the enhanced uptake of FDP-glucose by tumour cells, PET is able to detect both local recurrence and distant metastases. However, limitations of PET scan include the inability in identifying small volume disease [92] and a relatively low sensitivity for detecting lymph node metastases [92]. In addition mucinous adenocarcinomas have poor FDG uptake [93] and therefore can be easily missed by PET scan.
Imaging to Exclude Distant Metastases
Accurate identification of extrapelvic disease is key for the decision to operate a patient. CT and MRI have demonstrated high sensitivity in detecting distant recurrence. Both imaging modalities can provide at the same time detailed anatomical information of the affected organ and tumor extension into surrounding tissues [61, 98]. The accuracy of CT in detecting abdominal disease has been demonstrated to be over 85 % [61] with the MRI’s accuracy ranging to similar levels [64, 65].
A meta-analysis that investigated the value of US, CT, MRI and PET in detecting liver metastases, demonstrated a sensitivity of 63, 74.8, 81.1 and 97.2 % respectively and specificities of more than 93.8 %, with MRI being significantly more sensitive than CT (p = 0.05) and equally sensitive to PET (p = 0.02) [99]. There were no significant differences in the sensitivity between PET and CT (p > 0.05) and neither between CT and US (p = 0.45) [99].
Positron emission tomography (PET) has been demonstrated to be highly accurate in the detection of disseminated disease [100–103] and to have significant impact on the management of patients with suspected recurrent colorectal cancer [104, 105]. A meta-analysis reported a PET sensitivity of 91 % and specificity of 83 % for the diagnosis of distant metastases [90]. However the authors admitted that only 8/27 (29.6 %) studies were of high quality fulfilling their quality criteria at least by 80 %. Another study showed that the overall added value of PET in the management of patients with local and/or distant recurrent colorectal cancer is 8 % and suggested that PET should be used when findings remain equivocal after serial imaging review [106]. In the authors practice, all patients with locally advanced primary and recurrent rectal cancer that have potentially resectable local disease undergo a PET scan to exclude distant disease.
Selection Criteria for Surgery
Decision for surgery is made after extensive discussions at the local multidisciplinary meeting (MDM) and heavily depends on the findings of the available diagnostic modalities. Based on the radiological findings a decision will be made regarding the tumor resectability. Therefore accurate preoperative staging in extremely valuable in this group of patients as it can help to establish the extent of local disease and the presence or absence of distant metastases and therefore influence the outcome of the MDM.
Distant Recurrence
The presence of distant metastases is normally considered as a contraindication to proceed for surgery [107]. However, a number of centers have demonstrated that synchronous or staged resection of locoregional recurrence and distant metastases can have acceptable results in highly selected patients [108–110]. It is generally considered a contraindication though, due to the significant morbidity that is may be associated with this type of procedures [23, 29, 111–113].
Resectable Local Recurrence
In the absence of distant disease, surgical resection of the primary cancer or the locoregional recurrence is the only potentially curative option. Surgery for advanced primary or recurrent rectal cancer includes a range of different procedures that depend on the extent of the disease and the specific organs/structures that are involved. Surgery has to be performed en bloc and is considered curative when the resection margins are free of microscopic disease (R0 resection). The presence of microscopic or macroscopic residual disease at the resection margins is defined as R1 and R2 resection respectively. It has been previously demonstrated that R1 or R2 resection can result in poorer survival [18, 114–116] and it should be consequently considered as palliative resection. A recent study showed that patients that undergo an R2 resection have similar oncological outcome with the patients that receive palliative chemotherapy [117]. Considering that this type of surgery carries considerable mortality and morbidity, identification of patients that an R0 resection can be potentially achieved is crucial and extremely difficult. Preoperative imaging with PET, CT and MRI and clinical assessment are utilized in an effort to optimize the selection of patients in whom curative resection is considered possible as well as those in whom curative resection is an unlike scenario.
Contraindications for Surgical Resection
One of the key factors that guide patient management is the patient’s fitness for surgery. It is essential to assess it prior to any discussion for surgical options since the lack of fitness is often considered a contraindication when undergoing such a major procedure, due to the significant risk of death and complications. Operation is contraindicated in the presence of circumferential or extensive lateral pelvic sidewall involvement, involvement of the iliac vessels, bilateral ureteric obstruction, sciatic nerve involvement and periaortic lymph node metastases [26, 107, 108, 118, 119]. Involvement of the external iliac vessels may present with lower limp edema whereas ureteric obstruction with hydronephrosis. Tumor invasion of the sciatic nerve may present with lower limp pain and weakness. Limited tumor invasion to the lateral pelvic sidewall and invasion of the sacrum above the S2 vertebrae are considered relative contraindications since there are surgical options in both cases [23, 29, 120]. However the likelihood of a complete resection is considerably low while the perioperative risk of mortality and morbidity is higher.
Irresectable Local Recurrence
Surgical resection and chemoradiotherapy can be used for palliation, alleviating the patients’ symptoms that are related to the organs/structure that are invaded by tumor (Fig. 10.3). It has been suggested that palliative resection can have an improvement in quality of life and pain relief [121, 122]. However its use can be usually unsuitable considering the co-morbidities related with this type of surgery [123]. It is therefore important that the patients are carefully selected for palliative procedures taking into consideration possible co-morbidities and their social circumstances, as the benefits from these procedures are short term. The symptomatic relief can last up to 17 months with median symptom free interval of 4 months compared with 23 months for non-palliative procedures (p < 0.001) [124].