Fig. 11.1
(a) Plane of resection utilized when TME plane is followed to its termination. A waist is produced at the level of the puborectalis. (b) Plane of resection when the abdominal dissection is terminated at the level of the pelvic floor and the levators divided at the linea terminalis
To achieve a ‘cylindrical’ specimen, the abdominal dissection is halted above the pelvic floor at the level of the sacrococcygeal joint, just below the hypogastric nerves laterally and at the lower border of the seminal vesicles or uteri cervix anteriorly. The perineal dissection is commenced via a perianal incision of varying width according to local tumor extent, continues in the ischioanal fat outside the sphincter to the levator muscle insertions on to the pelvic side wall. In the modern iteration the coccyx is disarticulated in some cases where exposure is required (more often needed in a prone position). The levator plate is divided widely from posterior to anterior (if prone and the reverse if supine) and the specimen carefully exteriorized allowing dissection off the posterior aspect of the vagina or prostate.
Wide dissection of the pelvic floor avoids waisting of the specimen but does commonly result in perineal wound complications, even with xeno- or local tissue grafts/flaps [25]. Although general quality of life may not be impaired, perineal wound problems and pain are commonplace alongside urinary and sexual dysfunction [26].
The necessity of such an aggressively wide dissection in all comers has been questioned [27]. A comparative Swedish study comparing 79 consecutive ELAPE operations with 79 “standard APR” historical controls (performed by the same surgeons before and after a ELAPE training conference) showed no significant difference in CRM involvement, tumor perforation, or local recurrence rates between groups. However wound complications were substantially higher with a longer length of hospital stay in those undergoing ELAPE [28].This was mirrored by a study from the Mayo clinic in 2012 in which 246 patients underwent APR in the Lloyd-Davies (supine) position [29]. The local recurrence rate at 5 years was 5.5 %, not significantly different from that after AR. Furthermore, disease-free survival was the same after APE and AR. A recent systematic review demonstrated no significant difference between ELAPE and standard resections. There was no evidence that extralevator abdominoperineal excision yielded significantly lower rates of resection margin involvement or intra-operative bowel perforation compared with standard abdominoperineal excision in six independent hospital- and population-based patient series [30].
The current debate highlights a failure to communicate effectively and the importance of definitions and terminology, or rather a disabling lack of defined, standardized, internationally, and temporally acceptable terms of reference. Somewhere in the last century we left Miles behind and forgot his principles – or did we? Patients undergoing “standard” APR actually had the levators taken en bloc from the pelvic side wall thereby avoiding Morson’s waist suggesting that appropriately trained, specialist surgeons already included levator excision in the AP resection of a rectal tumor (as described by Miles). Notwithstanding this, moves across Europe continue to promote ‘extralevator’ AP excision with formal workshops and training programs such as LOREC [31]. Hopefully such initiatives will serve to emphasize appropriately careful operative technique, standardized boundaries, and macroscopic margin targets of the current procedure.
Laparoscopic Abdominoperineal Resection (LAPR)
Laparoscopic APR (LAPR) represents a truly laparoscopic procedure with specimen extraction through the perineum. Perceived technical difficulties in the deep pelvis excluded rectal cancer patients from many of the initial laparoscopic surgery trials. It is speculated that the learning curve for laparoscopic rectal resection may be greater than 70 procedures [32]. Furthermore the oncological safety of LAPR has been questioned with a CRM positivity rate of up 16 % quoted in some studies [33]. However in these series, surgeon persistence in following the TME plane to its termination above the anorectal ring rather than the technique of laparoscopy per say appears to be the causative problem.
A number of randomized controlled trials (RCT) have now produced mature data on LAPR. Both Ng’s RCT and subset analysis of the CLASICC trial showed that outcomes following laparosocpic APR were comparable to those following open resection [34, 35]. Postoperative recovery, return of bowel function and mobilization were quicker with lower analgesia requirements for patients undergoing LAPR but at the expense of longer operating time. Oncological outcomes and overall 5 year survival were equivalent [34, 35]. This is on the background of surgeons operating early on the learning curve with an associated high conversion rate (30.4 % in the CLASICC trial). A recent meta-analysis confirmed no difference in long term or oncological outcomes between open and LAPR while LAPR was associated with fewer short term complications (OR 2.159, 95 % CI 1.426–3.269, P = 0.000) [36]. Indeed there was some suggestion that local and distant recurrence rates were actually lower with LAPR (odds ratio 2.736 and 1.994, 95 % confidence interval 1.137–6.584 and 1.062–3.742, P = 0.025 and P = 0.032, respectively) [36]. Hand assist or hybrid approaches have also been applied to APR and serve as a bridge to total LAPR, combining laparoscopic colon mobisation with the benefits of a shorter abdominal incision and manual tactile sensation for traction/counter traction [37].
APR has also been performed using various single port platforms with the device being inserted at the colostomy site [38–40]. However evidence for this technique is confined to small case series involving highly selective patient cohorts with only short term follow up. Stewart et al. involved six patients with a median BMI of 28, LN yield of 18 and negative CRM in all cases. All specimens were removed through the perineum [38].
Robotic APR (RAPR)
Robotic surgery has been applied to APR [41– 43]. The erogonomics of the robotic systems may facilitate dissection of a large low neoplasm in an obese patient with a narrow pelvis thereby reducing conversion rates (Fig. 11.2) [41] and could theoretically translate into lower incidences of perioperative complication. It has been suggested that robotic APR (RAPR) may confer the benefits of laparoscopy while avoiding the prolonged learning curve associated with LAPR [42]. Straight and side docking techniques are routinely utilized (Fig. 11.3) [41, 42]. The currently available data suggest that surrogate oncological markers such as mesocolic dissection grade, lymph node number, perforation rates and margin involvement are equivalent for open, laparoscopic and robotic APR [43].
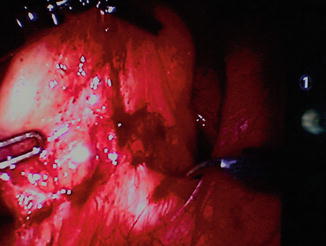
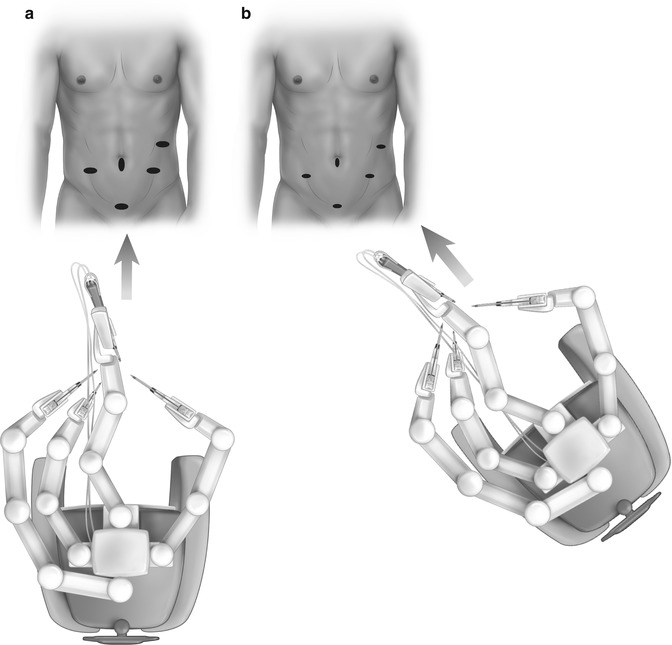

Fig. 11.2
Four articulated instruments easily accommodated in narrow pelvis of patient undergoing RALR for a large low rectal tumor (ypT3N1)

Fig. 11.3
End (a) and side (b) docking for RAPR
At present, the financial costs associated with the robotoc approach prohibit its wider application to APR.
Surgical Technique
Open APR
Pre-operatively all patients are sited for an end colostomy and receive stoma education. Some surgeons favor a mechanical bowel preparation (either oral laxative solution or enema) with or without oral antibiotics. Prophylactic intravenous antibiotics are administered at induction and a urinary catheter then inserted. Care must be given to appropriately pad pressure areas and potential nerve entrapment points. Neuropraxia has been reported after prolonged lithotomy [44] positioning but this was prior to widespread use of padded boots (rather than metallic stirrups) and sequential compression devices for enhanced venous blood flow and reduction of thrombotic events. After standard skin preparation, the peritoneal cavity is accessed via a lower midline infra-umibilical incision for open surgery or direct vision blunt port access for a laparoscopic approach. The sigmoid colon is mobilized beginning laterally at Todlt’s line allowing medialization of the left colon to its embryonic midline position. Care is taken to protect the left ureter and gonadal vessels and maintain an intact mesocolic into mesorectal envelope by utilizing the natural fusion plane between the mesocolon and Toldt’s fascia. The inferior mesenteric artery is ligated or vessel sealed within 1 cm of its origin to ensure all draining lymph nodes are harvested (while preserving the sympathetic hypogastric plexus) for histological staging. Alternatively, the ‘medial to lateral’ approach is used with the same steps in reverse order. There is little advantage to this and ureteric visualization is not as efficient.
Division of the mesocolon is begun proximal to the IMA and carried laterally towards the junction of the sigmoid and descending colon. The left colic artery and inferior mesenteric vein are sequentially secured as encountered. The colon is then divided after ensuring pulsatile blood flow in the marginal artery and adequate tension free length to permit end colostomy formation.
Sharp dissection is continued posteriorly, in the areolar tissue plane between the parietal pelvic wall and the visceral endopelvic fascia of the rectum, thereby ensuring an intact mesorectal envelope. The rectosacral (Waldeyer’s) fascia is sharply divided and the dissection is continued to the level of the sacrococcygeal junction. Care is taken to maintain the correct plane and avoid potentially torrential bleeding from the presacral venous plexus. Laterally the ureters are protected on the pelvic side wall and dissection is terminated just below the nervi ergentes. The lateral stalks representing condensation of the endopelvic fascia running in a posterolateral direction are divided. The peritoneal reflection is then identified and entered. The dissection is continued posterior to Denonvilliers fascia unless the tumor is anteriorly located. Recent work has demonstrated that Denonvillier’s fascia is a distinct embryological entity representing a condensation of the parietal endopelvic fascia separate to the rectal mesocolic fascia. This serves to protect branches of the inferior hypogastric plexus passing to the pelvic urogenital tract. This anterior dissection is stopped at the uteri cervix in females or lower border of the seminal vesicles in males. The bowel is now divided at the junction between the sigmoid and descending colon. If the patient is to be turned prone for the perineal phase (or if the procedure a laparoscopic one), the end colostomy is fashioned at the marked site in a standard fashion.
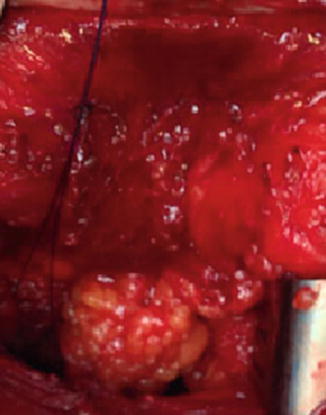
The perineal dissection may be performed in the prone or lithotomy positions according to surgeon preference without detrimental consequences to oncological outcomes. The anal canal is ideally closed with a purse-string suture at the beginning of the operation prior to rectal mobilization to prevent spillage. An elliptical skin incision around the anal canal should be wide enough to include the sphincter and any local tumor – hence it is tailored to the tumor size, level, and extent (Fig. 11.4). The dissection is carried through the ischioanal fossa to the undersurface of the levator plate. The inferior rectal artery arising from the internal pudendal artery in Alcock’s canal is encountered running from lateral to medial. It’s origins from the linea terminales of the posterior obturator fascia are now circumferentially exposed. The coccyx is identified and the anococcygeal ligament is divided if necessary to facilitate coalescence of the abdominal and perineal dissections. The levator muscles are now divided laterally to join the abdominal dissection plane. The specimen is carefully exteriorized and the dissection continued anteriorly off the posterior aspect of the prostate or vagina, taking care to preserve the neurovascular bundles. This phase can be completed with the rectum in position when the specimen is too bulky to evert (Fig. 11.5). Hemostasis is secured and surgeons should be aware of an aberrant obturator artery which can cause troublesome bleeding in the small but appreciable proportion of patients who have one.
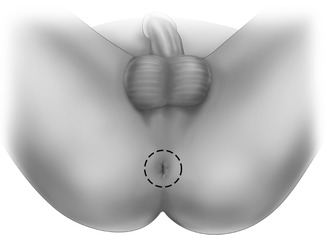
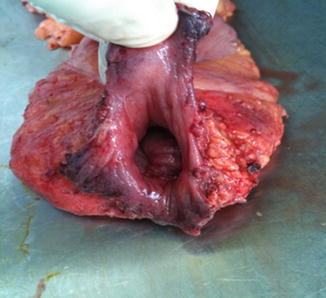

Fig. 11.4
Perineal skin incision for perineal phase of contemporary APR

Fig. 11.5
Operative specimen for perineal phase of contemporary APR with the levators divided at their origin from the pelvic side wall. Note no evidence of Morsons waist at the level of the puborectalis
If present and mobile, the uterus can be retroflexed to facilitate pelvic closure. The authors routinely use omental pedicled flap to fill the pelvic dead space and aide primary perineal wound closure [45]. This is done in layers with 2/0 polydioxanone sulfate or equivalent. The perineal skin is closed with 3/0 or 2/0 non-absorbable interrupted vertical mattress sutures (or subcuticular absorbable suture if the wound is small, clean, and non-irradiated). A closed suction pelvic drain (placed from the abdomen or perineum) is helpful in maintaining a dry perineal wound as a seroma is a common cause of wound failure without one but they are uncomfortable for patients. It is recognized that many centers routinely use a local (buttock) or distant (rectus abdominis muscle) flap, porcine/bovine dermis graft, or even their combination for closure of the perineum.
Laparoscopic APR (LAPR)
The patient is positioned as for open surgery but with an inflatable bean bag, gel mat or “mummy wrap” used to ensure the patient remains securely fixed to the operating table for steep Trendelenburg with lateral tilt for prolonged periods. Fears regarding intraocular pressure elevations have been raised but not substantiated by adverse events. The authors technique involves the surgeon and camera person on the patient’s right side with an assistant on the patient’s left. Camera monitors are placed at the patient’s feet and left side.
The authors favor a 3 or 4 port technique (Fig. 11.6a, b) with a 5 mm camera port at or above the umbilicus. The right iliac fossa (RIF) port is a consistent operating port while the suprapubic and LIF port alternate between the surgeon and assistant. The patient is initially placed in steep Trendelenburg position and mobilization commenced lateral to medial or medial to lateral as described for open surgery. The ureter and gonadal vessels are identified and preserved. The IMA is safely sealed with an energy device unless the vessel is heavily calcified when clips or locking grips may be utilized as adjuncts or the primary tools. The lateral colonic attachments are now divided. The splenic flexure may be mobilized if there is insufficient length for a colostomy (rarely needed). At this stage the authors routinely mobilize the omentum (usually on the gastroepiploic arcade) for perineal omentoplasty later in the operation. An accessory port at the proposed stoma site is helpful in providing counter traction (a 12 mm port is used to accommodate an endostapler for colonic division). In females it may be necessary (or helpful) to hitch up the uterus using an externally tied suture though the broad ligament. The dissection is as for open surgery and the dissection is stopped above the levator plate. At this stage the colon is divided with an endostapler and a colostomy formed at the premarked site. Again, the perineal phase may be performed in the supine position or the patient may be turned prone depending on surgeon preference.
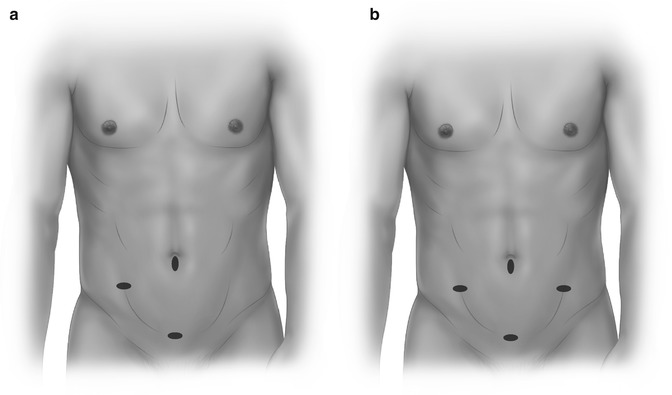

Fig. 11.6
Three-port (a) and four-port (b) configuration for LAPR
Controversies
Patient Positioning for APR
A central tenet of recently published APR operative series is prone positioning. The prone position does afford excellent visualization, particularly during the potentially hazardous anterior mobilization of the specimen from the prostate/vagina and may facilitate nerve preservation. Prone positioning is however time consuming and can pose an anesthetic challenge. Furthermore, a number of groups have demonstrated that step wise or synchronous APR in the lithotomy or modified Lloyd-Davis position is oncologicaly safe with equivalent short and long term outcomes [46, 47]. A direct comparative study from the Cleveland Clinic involving APR performed in 81 patients in the prone position and 87 patients in the supine position showed no difference in local (equivalent at 5.7 and 12.5 % for supine and prone APR respectively) or distant recurrence (equivalent at 20 % after 7 years follow up) or overall survival (62.5 and 59.4 % 5 year survival). The patient groups were equivalent for demographic profile, use of adjuvant/neoadjuvant therapy, and tumor stage [46].
Additionally, a national multi-institutional study of standardized APR performed in the supine position demonstrated a local recurrence rate of 6.0 % at 5 years. It concluded that in patients undergoing APR by appropriately trained surgeons using a standardized approach, margin positivity was dictated by tumor stage, but not by center or surgeon [47]. In our opinion, patient position is at the surgeons discretion provided the resection is performed in a standardized manner incorporating en-bloc the pelvic floor and rectum.
Perineal Reconstruction
Short and long term perineal wound complications are common following abdominoperineal resection. Regardless of approach (open, laparoscopic, robotic, lithotomy or prone), anorectal resection produces a large fixed dead-space cavity which accumulates fluid and blood clot, promoting pelvic and perineal sepsis, abscess formation and ultimately delayed wound healing. The consequences include prolonged hospital stay, increased readmission rates, increased nursing home care requirements with resultant patient and societal financial expense [48]. Higher perineal morbidity has been described following the more radical ELAPE [26]. Primary healing rates range from 45 to 91 % depending on the study, population, concomitant use of neoadjuvant radiotherapy/chemo-radiotherapy and surgical technique. Careful management of the perineal wound and pelvic cavity post APR is thus vital [48, 49]. Historically the perineum was left open and packed following APR to promote hemostasis and drainage with subsequent healing by secondary intention. Wound healing was often delayed beyond 4 months causing considerable patient discomfort [50].
Management strategies for the pelvic and perineal defects following APR have evolved to include primary perineal wound closure, closure of the peritoneum, primary closure with closed suction drainage of the pelvis drainage and pelvic wound irrigation and active closed drainage [51, 52]. Peritoneal and perineal closure was associated with fluid accumulation and subsequent infection in the dead space collection beneath the peritoneum and this technique thus fallen out of favor. The addition of irrigation to the pelvic drainage was not shown to be beneficial in an RCT from 1991 [52].
While primary perineal wound closure and closed suction is the preferred method following APR, a variety of complex, costly and time-consuming wound management techniques incorporating tissue transfer, such as vertical rectus abdominis (VRAM) and gracilis flap construction, have been advocated to deal with the pelvic cavity dead space and aide wound apposition [53–55]. Prophylactic biological matrix insertion has also been employed in an attempt to circumvent these difficulties [56]. Not all patients undergoing APR require such radical supplemental tissue transfer techniques. A very extensive resection for locally advanced rectal neoplasms with perianal skin involvement mandates formal myocutaneous grafting [57]. However the authors favor the use of omentum after APR. The anatomical, physiological and immunological properties coupled to availability of the omentum, especially laparoscopically, make it an excellent candidate for pedicled transfer to the pelvis [45]. Such a flap has sufficient length to reach the pelvic floor and adequate mass to fill any dead space (Fig. 11.7). A recent systematic review has shown that omental flap transfer and buttressing of the primary perineal repair following APR reduces wound infections, reoperation rates and hospital length of stay with minimal additional operative time or flap-associated morbidity [58].