Overall Bottom Line
- Due to long-term immunosuppression therapy, the post-LT recipient is at risk for disease conditions (e.g. infection, malignancy) that are generally regulated by the body’s immune system.
- Skin cancer is the most common type of de novo malignancy after LT.
- Malignancy, either de novo type or recurrent HCC, may occur after LT.
- PTLD is a malignancy unique to the transplant recipient.
- Specific screening guidelines have not yet been established for LT recipients; the current ones for immunocompetent persons remain in use. Increased surveillance may be prudent in view of the recipient’s immunosuppressed state.
- Treatment can be tailored according to the particular tumor, along with reduction of the immunosuppression regimen to strengthen the individual’s immune system.
- Molecular markers may shed more light in the future on risk estimation of HCC recurrence post-transplantation.
Section 1: Background
Definition of disease
- De novo malignancy is the second cause of mortality after LT, with cardiovascular disease as the primary reason; cumulative incidence ranges up to 26%.
Incidence/prevalence
- There is a higher incidence of developing malignancy in post-transplant (i.e. immunosuppressed) recipients when compared with the immunocompetent population.
- The cumulative incidence of de novo malignancies range up to 26%, with 0.5% for the general non-transplant population.
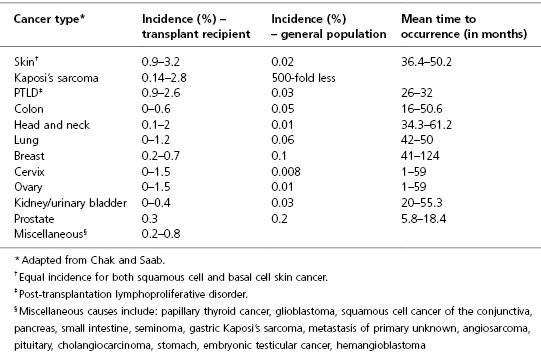
Comparison of the incidence of the common types of malignancy after LT and the incidence in the general population
Etiology
- The etiology of malignancy after LT is related to long-term immunosuppression use.
Pathology/pathogenesis
- Immunosuppression results in the weakening of the body’s natural defenses (e.g. cytotoxic T cells, macrophages, natural killer cells) that generally inhibit oncogenic viral growth and destroy malignant cells in vitro.
- Azathioprine use is an independent risk factor for the increased incidence of de novo malignancies. By inhibiting purine synthesis, it affects T cell and B cell production.
Predictive/risk factors
- Smoking.
- Alcohol abuse pre-LT.
- Pre-malignant disease (age and gender factors have not been replicated in studies)
- Barrett’s esophagus.
- Myeloproliferative disorder.
- Cervical atypia.
- Colon polyps.
- Ulcerative colitis.
- Caroli disease (as a risk factor for cholangiocarcinoma).
- Barrett’s esophagus.
Section 2: Prevention
Bottom Line/Clinical Pearls
- Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor, and in a different drug category from tacrolimus and cyclosporine (calcineurin inhibitors). It has anti-angiogenic properties and has been reported to decrease skin cancer incidence in kidney transplant recipients.
- mTOR is upregulated in HCC, therefore allowing sirolimus to have a potential effect on HCC recurrence. A recent meta-analysis demonstrated that sirolimus use to reduce HCC recurrence rates post-LT resulted in a lower recurrence rate, longer recurrence-free survival and overall survival, and lower recurrence-related mortality when compared with patients receiving calcineurin inhibitors.
Screening
- There are currently no guidelines on the surveillance schedule for HCC post-LT.
- At Mount Sinai Hospital, the recipients’ explants are assessed and classified as either low-risk or high-risk for recurrence, depending on the HCC histology, number of tumors and presence/absence of lymphovascular invasion.
Surveillance imaging protocol for patients who undergo LT for HCC at Mount Sinai Hospital
| Imaging schedule* | Low-risk HCC | High-risk HCC |
|---|---|---|
| Year 1 | At 3 months post-transplantation | Every 3 months |
| Year 2 | At 12 months post-transplantation | Every 6 months |
| Year 3 | End | Every 6 months |
| Year 4 | N/A | Every 12 months |
| Year 5 | N/A | Every 12 months |
| Year 6 | N/A | End |
* Imaging studies: chest CT scan (without contrast) and abdomen CT scan or MRI (with intravenous contrast)
- Current guidelines for de novo malignancy screening have not yielded consistent benefits in both the liver and kidney transplantation populations to justify the cost-effectiveness of this approach, nonetheless various organizations recommend screening guidelines for the average-risk individual.
| Organ/cancer type | Screening guidelines for average-risk individuals | |
|---|---|---|
| Onset | Interval | |
| Breast | Starting age 50 | Between 12–24 months |
| Cervix | Between ages 18 and 20, or when sexual activity begins | Annually |
| Colon | Fecal occult blood test and flexible sigmoidoscopy starting age of 50 | Every 5 years |
| Skin | Self-examination | Monthly |
| Clinician examination | Every 6–12 months | |
| Kidney | No guidelines | N/A |
| Lung | No guidelines | N/A |
| Prostate | PSA and DRE starting age of 50 | Annually |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


