Fig. 4.1
The relative position of levator ani subdivisions during ultrasound imaging. Levels 1–3 are identified below the figure. The A–J markings on top of the figure correspond to the ultrasound images shown in Fig. 4.4. IC iliococcygeus, PP puboperinealis, STP superficial transverse perinea, PA puboanalis. Illustration: John Yanson. © Shobeiri. Ultrasonography Validation. Obstet Gynecol 2009
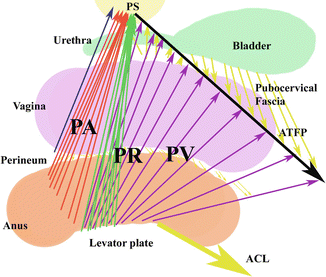
Fig. 4.2
Subgrouping of the pubovisceralis (PV), puborectalis (PR), and the puboanalis muscle groups. The lines of actions of these muscle groups and their relative contributions to the levator plate are shown. Anococcygeal ligaments (ACL), Arcus tendineus Fascia Pelvis (ATFP) are shown. © Shobeiri
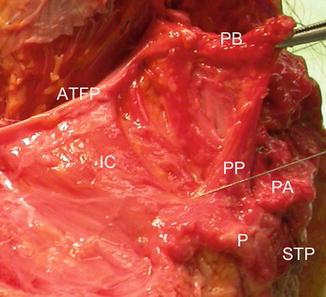
Fig. 4.3
Gross cadaveric dissection. A needle is seen inserted into the puboperinealis. PB pubic bone on pubic bone insertion, ATFP arcus tendineus fascia pelvis, IC iliococcygeus, PP puboperinealis, PA puboanalis, P perineum, STP superficial transverse perinei. Shobeiri. Ultrasonography Validation. Obstet Gynecol 2009
The pelvic floor muscles have the unique role of supporting the urogenital organs and the anorectum. Unlike most other skeletal muscles, the LAM maintains constant tone, except during voiding, defecation and a Valsalva maneuver [4]. At rest, the LAM keeps the urogenital hiatus closed, by compressing the vagina, urethra, and rectum against the pubic bone, and maintains the pelvic floor and pelvic organs in a cephalic direction (Fig. 4.2) [2]. Pelvic floor muscles are integral to pelvic organ support, and while functioning properly, provide support to the pelvic organs, keeping the ligament and fascial attachments tension-free.
During parturition, the LAM stretches beyond its limits [5, 6] in order to allow passage of a term infant (Fig. 4.4). Studies have shown that LAM injury occur in 13–36 % of women who deliver vaginally [7–9]. There are various definitions of levator ani injury, according to mode of assessment and imaging modality. Most authors have used avulsion of the muscles as the end point of the study. However, more recent in publication studies using 3D endovaginal ultrasonography have found that up to 50 % of women may have hematoma formation after first delivery (Fig. 4.5). Assessment of the levator muscles is essential for a complete understanding of pelvic floor anatomy abnormalities, as well as of pelvic floor dysfunction.
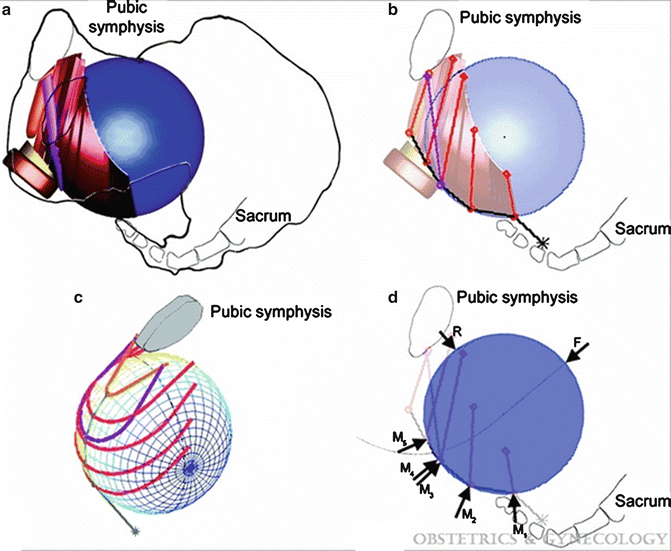
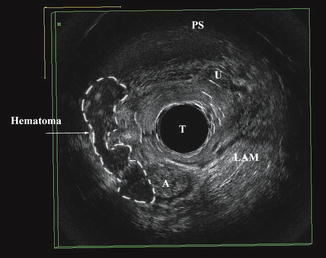

Fig. 4.4
(a) Initial geometry of the female pelvic floor at the beginning of the second stage of labor in a left lateral view. (b) Left lateral view of the pelvic floor model. (c) Left three-quarter view of the model. (d) A free body diagram of the model is shown in lateral view. (© Biomechanics Research Laboratory, University of Michigan). Lien. Efficacy of Maternal Effort. Obstet Gynecol 2009

Fig. 4.5
Hematoma formation in the right levator ani muscle territory is outlined. Anus (A), transducer (T), urethra (U), pubic symphysis (PS). © Shobeiri
4.1.2 3-D EVUS Technique for Levator Ani Imaging
All the endovaginal and endoanal images in this chapter are obtained from a Flex Focus (Fig. 4.6) or ProFocus Ultraview (Fig. 4.7) BK Medical scanner (BK Medical, Peabody, MA) as discussed in previous chapter on Instrumentations and techniques. For optimal images to be obtained, we recommend for the operator to have a clear understanding of the technique, as well as familiarity with the controls of the machine. Most importantly, improper settings of the equipment can lead to artifact.


Fig. 4.6
BK flex focus ultrasound machine with a 2052 probe

Fig. 4.7
BK ultrafocus ultrasound machine
Two 360° probes can be used for endovaginal levator ani imaging. The 2052 transducer (Fig. 4.8) is its built-in 3D automatic motorized system (proximal-distal actuation mechanism is enclosed within the shield of the probe). This equipment allows for the acquisition of 300 images in 60 s for a distance of 60 mm. The 8838 probe is a 60 mm 360° rotational transducer and obtains an image every 0.55° for a total of 720 images (Fig. 4.9). The images are acquired automatically with the touch of the 3D button on the equipment console. The data from the closely spaced 2D images are combined as a 3D volume displayed as a data volume which can then be stored and analyzed separately.



Fig. 4.8
BK 2052 transducer

Fig. 4.9
BK 8838 transducer
No special patient preparation is required and no vaginal or rectal contrast is necessary. The patient is asked to keep a comfortable amount of urine in the bladder. The patient is placed on the dorsal lithotomy position and the probe is inserted in a neutral position, with care not to press on the upper or lower vaginal areas so as not to distort anatomy. The probe should create a horizontal line with the body’s axis. When placing the ultrasound gel in the probe cover, we recommend for air bubbles to be gently squeezed out of the probe cover, so as to minimize the potential for artifact.
Once the 3D endovaginal imaging is selected on the console, the rotating crystal will begin to rotate, signaling that the probe is ready for insertion. The probe is inserted as described in endovaginal instrumentations and techniques chapter. Based on our anatomic studies, we recommend placing the probe 6 cm inside the vagina, just 2 cm above the level of the urethrovesical junction. If using the 2052 probe, the two buttons that move the crystal cephalad and caudad should be facing the 12 o’clock position. Once the acquisition is started, it is important that the operator minimize movement by stabilizing the probe during the full length of the scan. This will help optimize image quality in obtaining the 3D cube (Fig. 4.10). We have characterized 3 levels for assessment of the axial plane [3] (Fig. 4.1).

Fig. 4.10
An endovaginal 3D volume. © Shobeiri
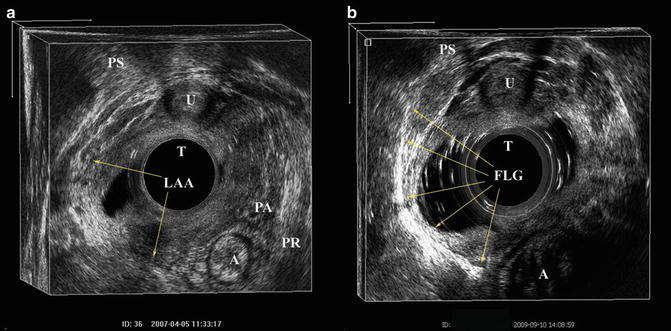
Level 1: Contains all the muscles that insert into the perineal body, namely the superficial transverse perinei (STP), puboperinealis, and puboanalis. The STP serves as the reference point.
Level 2: Contains the attachment of the pubovaginalis, puboperinealis, puboanalis, puborectalis, and iliococcygeus to the pubic bone.
Level 3: Contains the subdivisions cephalad to the inferior pubic ramus, namely the pubococcygeus and iliococcygeus, which wing out towards the ischial spine.
Functionally and based on the levator ani volume measurements, we divide the muscles into: (1) Puboanalis (Puboperinealis +Puboanalis), (2) Puborectalis, and (3) Pubovisceralis (Pubococcygeus + Iliococcygeus) (Fig. 4.2). By 3D endovaginal ultrasound reconstruction of nulliparous subjects, puboanalis, puborectalis, and pubovisceralis groups had the volume of 4.4 cm3 (Range 2.1–6.7 cm3), 4.2 cm3 (Range 1.9–6.5 cm3), 4.5 (Range 2.2–6.8 cm3) respectively. Although they have a wide range in volumes, the proportions remain constant within the individual [10].
When analyzing a 3D volume caudad to cephalad, the first structure to visualize as a landmark is the STP muscle (Fig. 4.11). Visualization of this structure will consistently point to the most caudad structure seen by the probe in the vaginal canal. In normal nulliparous individuals, the external anal sphincter may be visualized just below the STP. If using the 2052 probe, there are two buttons used to move the rotating crystal caudally or cephalad located on the dorsal portion of the probe handle. By pressing the cephalad button the rotating crystal can be slowly moved cephalad and the perineal body and puboperinealis muscle come to view (Fig. 4.12a, b). The puboperinealis is hard to find consistently for the untrained eyes because it perhaps has less than 30 muscle fibers and lies very close to the vaginal epithelium. At the same level but more laterally are the fibers of the puboanalis that travel at a 45° to surround the anal canal and insert into longitudinal fibers of the anus at the level of the external anal sphincter (Fig. 4.13). Continuing to move the crystal cephalad will show the puborectalis forming a sling around the rectum and it can be followed to its insertion into the inferior margin of the pubic symphysis and the perineal membrane. Moving further cephalad will show the medial relationship of the iliococcygeus muscle in its medial relationship to the puborectalis (Fig. 4.14).
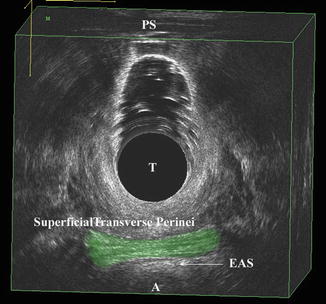
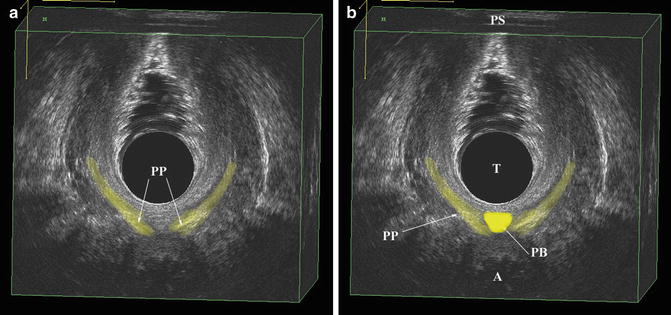
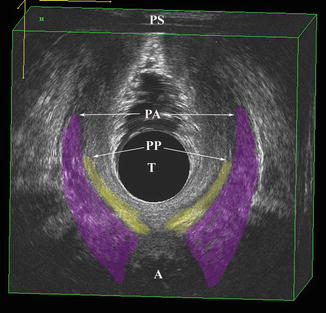
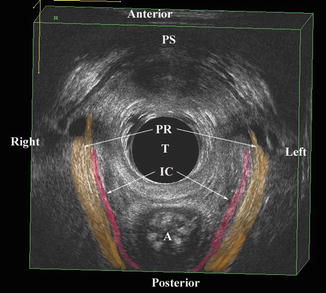

Fig. 4.11
The most caudad muscles seen by 3D endovaginal ultrasound imaging is the superficial transverse perinei muscle which is highlighted. External anal sphincter (EAS), anus (A), transducer (T), pubic symphysis (PS). © Shobeiri

Fig. 4.12
(a) The scant fibers of the puboperinealis muscles (PP) are highlighted. (b) The perineal body (PB) is highlighted in the same axial view as 3a. Pubic symphysis (PS), transducer (T), puboperinealis (PP), perineal body (PB), anus (A). © Shobeiri

Fig. 4.13
The puboanalis (PA) is shown at the same level as the Fig. 4.12. PA lies just lateral to the puboperinealis (PP) and they are part of the same functional groups. Anus (A), transducer (T), pubic symphysis (PS). © Shobeiri

Fig. 4.14
The puborectalis (PR) is shown at its cephalad insertion point to the pubic symphysis (PS). Note that PR has a wide insertion area which includes the PS, and the perineal membrane which is more caudad. The iliococcygeus muscle (IC) fibers are seen medial to the puborectalis fibers. Anus (A), transducer (T). © Shobeiri
The reliability of visualization of levator ani subdivisions have been reported in nulliparous patients. The levator ani subdivisions in these scans were examined at levels 1, 2, and 3 (Fig. 4.1). The visibility was scored by two blinded observers. Interrater reliability was calculated by taking the number of agreements and dividing by the number of observations in the total number of subjects. There was 98 %, 96 %, and 92 % agreement for level 1, 2, and 3 muscles respectively. Cohen’s kappa index/standard error were calculated for individual muscles as below: STP and puborectalis were seen by both raters 100 %, puboperinealis 0.645/0.2, pubovaginalis, and puboanalis 0.645/0.2 (95 % confidence interval 0.1–1), iliococcygeous 0.9/0.2 (95 % confidence interval 0.6–1).
In addition to the visualization of the muscle subdivisions, the interobserver and the interdisciplinary repeatability of (1) Levator hiatus length; (2) Levator hiatus width; (3) Levator hiatus area; (4) LAM attachment to the pubic rami, on both sides; (5) Anorectal angle (ARA); (6) Urethral thickness measurements using 3D endovaginal ultrasound have been established [11]. A team of six investigators of three different specialties (urogynecology—UGN, radiology—RAD, colorectal surgery—CRS) was formed. Each discipline included two investigators: UGN #1, UGN #2; RAD #1, RAD #2; CRS #1, CRS #2. Prior study initiation, a dedicated training session was completed and preliminary trial measurements were performed. For the training session, an expert 3D reader demonstrated to each of the readers the technique for measurements, including bony and soft tissue landmarks to be utilized. Readers discussed and refined the measurement technique for each parameter until all readers were in agreement regarding measurement methodology. In order to minimize the effect of imaging variations on the final measurements, a standardized protocol for review of the study datasets was strictly defined and jointly approved by all investigators.
Each ultrasound volume was displayed in a symmetrical orientation in the coronal, sagittal, and transverse planes and assessed in standardized sequences. The overall interobserver repeatability for levator hiatus dimensions was good to excellent (ICC, 0.655–0.889), for urethral thickness was good (ICC, 0.624), and for ARA was moderate (ICC, 0472) (Table 4.1). The interdisciplinary repeatability for levator hiatus indices was good to excellent (ICC, 0.639–0.915), for urethral thickness was moderate to good (ICC, 0.565–0.671), and for ARA was fair to moderate (ICC, 0.204–0.434) (Table 4.2) [11].
Table 4.1
Overall means and standard deviations (SD) of various measurements of individual readers
Observer | LH length (mm) | LH width (mm) | LH area (cm2) | Urethral thickness (mm) | ARA (degrees) |
|---|---|---|---|---|---|
UGN #1 | 50.42 (SD: 4.18) | 35.03 (SD: 3.50) | 10.48 (SD: 1.51) | 12.82 (SD: 1.6) | 133.1 (SD: 12.3) |
UGN #2 | 48.62 (SD: 4.87) | 34.21 (SD: 3.30) | 10.60 (SD: 1.31) | 13.06 (SD: 1.41) | 144.2 (SD: 7.03) |
RAD #1 | 48.71 (SD: 4.84) | 33.76 (SD: 3.50) | 10.72 (SD: 1.70) | 12.86 (SD: 1.73) | 143.04 (SD: 12.5) |
RAD #2 | 47.55 (SD: 5.62) | 33.54 (SD: 3.32) | 11.76 (SD: 1.35) | 12.61 (SD: 1.32) | 141.1 (SD: 7.99) |
CRS #1 | 47.95 (SD: 4.20) | 34.52 (SD: 3.38) | 10.82 (SD: 1.60) | 12.23 (SD: 1.77) | 143.8 (SD: 9.97) |
CRS #2 | 47.20 (SD: 4.05) | 34.06 (SD: 2.96) | 10.14 (SD: 1.60) | 12.30 (SD: 1.44) | 136.1 (SD: 5.94) |
Table 4.2
Interobserver, intra- and interdisciplinary repeatability of three-dimensional endovaginal ultrasound parameters
Repeatability | LH length | LH width | LH area | Urethral thickness | ARA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
ICC | 95%CI | ICC | 95%CI | ICC | 95%CI | ICC | 95%CI | ICC | 95%CI | |
Overall | 0.655 | 0.509–0.794 | 0.889 | 0.822–0.940 | 0.810 | 0.707–0.894 | 0.624 | 0.472–0.772 | 0.331 | 0.179–0.528 |
Intradisciplinary | ||||||||||
UGN #1 vs. UGN #2 | 0.643 | 0.359–0.819 | 0.889 | 0.773–0.948 | 0.857 | 0.713–0.932 | 0.660 | 0.385–0.829 | 0.035 | −0.339–0.402 |
RAD #1 vs. RAD #2 | 0.717 | 0.473–0.860 | 0.981 | 0.958–0.991 | 0.893 | 0.781–0.950 | 0.601 | 0.298–0.795 | 0.569 | 0.252–0.777 |
CRS #1 vs. CRS #2 | 0.883 | 0.761–0.945 | 0.910 | 0.815–0.958 | 0.887 | 0.770–0.947 | 0.735 | 0.501–0.869 | 0.216 | −0.167–0.544 |
Interdisciplinary | ||||||||||
RADs vs. CRSs | 0.677 | 0.514–0.815 | 0.915 | 0.855–0.956 | 0.831 | 0.724–0.909 | 0.651 | 0.482–0.798 | 0.434 | 0.241–0.639 |
RADs vs. UGNs | 0.639 | 0.467–0.790 | 0.897 | 0.826–0.946 | 0.851 | 0.755–0.921 | 0.565 | 0.380–0.739 | 0.327 | 0.139–0.549 |
UGNs vs. CRSs | 0.694 | 0.536–0.826 | 0.874 | 0.790–0.934 | 0.783 | 0.656–0.882 | 0.671 | 0.506–0.811 | 0.204 | 0.032–0.431 |
4.1.3 Clinical Applications
Pelvic floor disorders are common, costly, and distressing conditions for women resulting in greater than 300,000 operations per year, leading to considerable suffering from conditions not readily cured by surgery [12]. Fifty-five percent of women with pelvic organ prolapse (POP) have visible major LAM damage compared to 15 % of women with normal support making it the strongest known factor to be associated with both vaginal birth and POP [13]. The ability to diagnose injury to the LA muscle relies on advancements in imaging. Levator ani avulsion as imaged by transperineal ultrasound appears to double the risk of any significant anterior and central compartment prolapse [14].
4.2 Levaror Ani Injury
Recent literature has identified the distal subdivisions of the levator ani, classifying them based on attachment points, using magnetic resonance imaging. By MRI the LAM has been divided to pubovisceralis (to include pubovaginalis, puboanalis, puboperinealis, pubococcygeus and iliococcygeus) and puborectalis [1]. Morgan et al. have described levator ani defects and scored unilateral muscle defects separately [15]. The terminology for EVUS is different and in order to better functionally describe these LAM subdivisions, we group them as the puboanalis, puborectalis, and pubovisceralis (Fig. 4.2). Our technique and the anatomical descriptions were first authenticated in female cadavers and then in live human female volunteers, documenting superior, dynamic imaging, and visualization of these structures [3]. LAM injury has been described on MRI studies as a “defect” or “avulsion,” attributed to causes such as obstetric factors, aging or hormonal changes. 3D endovaginal ultrasonography has been used for visualization of the levator ani avulsion before and after bridging repair using fascia lata graft [16] (Fig. 4.15). This repair was done remote from delivery due to the patients’ symptoms. The goal of pelvic floor reconstruction is to restore the anatomy and hope that will translate into restoration of physiology and ultimately improve the patient’s symptoms. To restore the normal anatomy, if we repair the LAMs, normal functioning of the muscles may resume as long as the innervations is intact. Current “routine” surgical practice does not address these defects, but the sequalea of pelvic floor injury appears years after its occurrence. There is still the question of whether the identification and repair of these muscles early on will spare the patient from future POP and incontinence. Since identification of the muscle fibers is difficult without a localization technique, attempts at repairing these muscles without intraoperative visualization may have questionable results. In the absence of symptoms prompting LAM repair, it will require a large cohort and long term follow-up to determine if preemptive repair of the LAMs will translate into reduction of incontinence or POP [17]. In a case of bilateral levator ani injury (Fig. 4.16) after vaginal delivery, a patient underwent 3D endovaginal ultrasound and under ultrasound guidance (Fig. 4.17), the detached levator muscles were tagged with J-hook needles (MPM Medical, Elmwood Park, NJ) bilaterally. The needle could be manipulated to identify the torn ends of muscles. A vertical incision was made on the lateral wall of the vagina cephalad to the puboperinealis muscle which is the muscle traversing between the pubic symphysis and the perineal body. The dissection was made laterally to reach the area of needle. The tissue was grasped and then, with a finger in the rectum to ascertain rectal elevation, 2.0 vicryl sutures were passed (Fig. 4.3), 1 cm apart, and these were brought anteriorly to the level of the arcus tendineous insertion into the pubic bone under direct visualization and tied sequentially. The procedure was repeated on the contralateral side with palpable lift of ARA.