Table 23.2 Treatment recommendations for gastric adenocarcinoma
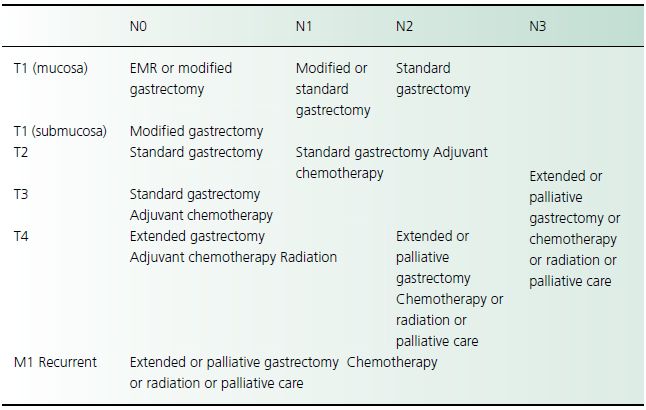
Surgical therapy
Complete surgical resection is the only therapy that offers a potential cure for gastric adenocarcinoma but the advanced stage at which more than half of patients present precludes curative surgery. The importance of surgical resection is reflected in the 5-year survival rate of 35–45% of patients with resectable tumors, compared with the 5-year survival rate of less than 5% of patients who undergo palliative resection.
Although surgery is recognized as the best treatment option for gastric cancer, there is little consensus on the optimal curative surgical procedure for gastric adenocarcinoma, especially concerning the extent of lymph node dissection. Adenocarcinomas of the proximal fundus are treated by proximal gastric resection. Tumors of the gastroesophageal junction require en bloc resection of the distal esophagus and proximal stomach, often by a combined thoracic and abdominal approach. Splenectomy usually is performed if tumors are located along the greater curvature. The role of resection of isolated hepatic metastases at the time of gastrectomy has not been determined in controlled clinical trials. Palliative surgery may be indicated for obstruction, perforation, or bleeding. Bypass procedures provide significantly shorter periods of palliation compared to resection.
Endoscopic procedures
Endoscopic submucosal dissection (ESD) has been shown to cure early gastric cancer in Japanese populations. Palliative endoscopic therapy may consist of stent placement or Nd:YAG laser tumor ablation, both of which may be used to treat obstruction. Gastrointestinal hemorrhage may be controlled by Nd:YAG laser coagulation necrosis.
Medical therapy
The role of chemotherapy and radiation therapy in treating gastric adenocarcinoma is evolving. Agents shown to decrease tumor mass include 5-fluorouracil, mitomycin C, doxorubicin, cisplatin, and hydroxyurea but there is no evidence of improved survival. Postoperative radiotherapy has likewise not been shown to increase survival.
- A Virchow node indicates metastasis to the left supraclavicular lymph node.
- A periumbilical nodule (Sister Mary Joseph node) may indicate tumor spread along peritoneal surfaces.
- Gastric cancers are associated with paraneoplastic syndromes, such as acanthosis nigricans, membranous glomerulonephritis, microangiopathic hemolytic anemia, arterial and venous thrombi (Trousseau syndrome), seborrheic dermatitis (Leser–Trélat sign), or dermatomyositis.
- Esophagogastroduodenoscopy (EGD) with biopsy is necessary to confirm the diagnosis.
- EUS provides accurate local and regional staging (T and N stages) but CT scan should be performed to assess for metastatic disease.
- The two best predictors of survival are depth of invasion (T stage) and metastases to lymph nodes (N stage) or distant sites (M stage).
Gastric Lymphoma
Clinical presentation
The presentation of gastric lymphoma is similar to that of gastric adenocarcinoma. Nonspecific symptoms include epigastric pain, weight loss, nausea, vomiting, early satiety, and anorexia. Gastrointestinal hemorrhage and perforation from extensive ulceration are less common manifestations. Physical examination may reveal an abdominal mass or peripheral adenopathy.
Diagnostic investigation
Upper gastrointestinal endoscopy and upper gastrointestinal barium radiography are the primary means for detecting gastric lymphoma; however, the ability to obtain biopsy specimens makes upper gastrointestinal endoscopy the procedure of choice. CT scans are required to determine extragastric involvement. Occasionally, lymphoma appears as a thickened fold on endoscopy and biopsy reveals a submucosal mass with normal overlying mucosa. In this setting, EUS can delineate which layers of the gastric wall are involved, and cytology or biopsy may confirm the diagnosis. Laparotomy may be necessary to define the extent of disease.
Management and course
Gastric lymphoma has a favorable prognosis compared with gastric adenocarcinoma. The 5-year survival rate is 50%. The Ann Arbor staging system for gastric lymphoma is based on the extent of disease which, once established, determines the appropriate course of management (Table 23.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




