Hemodialysis, hemoperfusion, and peritoneal dialysis (PD), particularly the first two procedures, can be useful adjuncts in the management of drug overdose and poisoning. However, these treatments should be applied selectively, as part of the general approach to the poisoned patient, which include supportive therapy, decontamination, elimination enhancement, and antidotes (Kulig, 1992). A review of data from the 2012 report of the American Association of Poison Control Centers shows that MDAC (multiple-dose activated charcoal) and alkalinization treatments far outnumber treatments by hemodialysis, and these in turn far outnumber treatments using hemoperfusion, with only 61 hemoperfusion treatments being reported versus 2,324 treatments using dialysis (Mowry, 2013).
I. DIALYSIS AND HEMOPERFUSION
A. Indications. Extracorporeal techniques should be considered when the conditions listed in Table 20.1 apply. Any procedure used in poisoning treatment should have a greater effect on drug elimination than that which occurs spontaneously. Early use of dialysis or hemoperfusion can be considered if the serum levels of a drug or poison are found to be increased to values known to be associated with death or serious tissue damage. Critical serum concentrations for several drugs are listed in Table 20.2. The information given in Tables 20.1 and 20.2 only represents a set of recommendations; the decision to institute dialysis or hemoperfusion must be made on an individual basis. In addition to providing extracorporeal drug elimination, dialysis can provide essential supportive care to poisoned patients with multiorgan or kidney injury. The EXTRIP (EXtracorporeal Treatment In Poisoning) work group is currently drafting guidelines for the use of blood purification in the context of overdose. Their publication should help to standardize management for these complex patients (Lavergne, 2012).
B. Choice of therapy
1. Peritoneal dialysis is not very effective in removing drugs from the blood, with maximal poison clearance rarely above 15 mL/min (about one-tenth of what usually can be reached with hemodialysis). Nevertheless, when hemodialysis is difficult to institute quickly, such as in small children, a prolonged session of PD can be a valuable adjunctive treatment for poisoning. Also, under certain conditions, such as in the hypothermic-poisoned patient, PD may be useful, as it may also be used to help in core rewarming.
Criteria for Consideration of Dialysis or Hemoperfusion in Poisoning | |
1. Progressive deterioration despite intensive supportive therapy
2. Severe intoxication with depression of midbrain function leading to hypoventilation, hypothermia, and hypotension
3. Development of complications of coma, such as pneumonia or septicemia, and underlying conditions predisposing to such complications (e.g., obstructive airways disease)
4. Impairment of normal drug excretory function in the presence of hepatic, cardiac, or renal insufficiency
5. Intoxication with agents with metabolic and/or delayed effects (e.g., methanol, ethylene glycol, and paraquat)
6. Intoxication with an extractable drug or poison, which can be removed at a rate exceeding endogenous elimination by liver or kidney
Serum Concentrations of Common Poisons in Excess of Which Hemodialysis (HD) or Hemoperfusion (HP) Should Be Considered | |
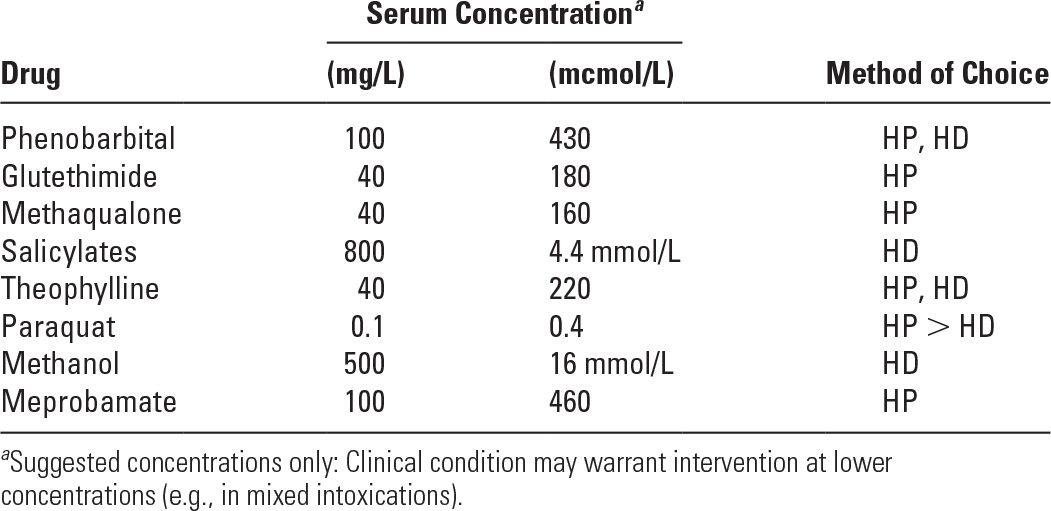
2. Hemodialysis is the therapy of choice for water-soluble drugs, especially those of low molecular weight along with a low level of protein binding, as such compounds will diffuse rapidly across the dialyzer membrane. Examples are ethanol, ethylene glycol, lithium, methanol, and salicylates. Water-soluble drugs that have high molecular weights (e.g., amphotericin B [MW 9,241] and vancomycin [MW 1,500]) diffuse across dialyzer membranes more slowly and are less well removed; removal rate is accelerated by use of high-flux membranes and hemodiafiltration. Hemodialysis is not very useful in removing lipid-soluble drugs (e.g., amitriptyline) with large volumes of distribution or drugs with extensive protein binding.
3. Hemoperfusion is a process whereby blood is passed through a device containing adsorbent particles. Most commonly, the adsorbent particles are activated charcoal or some sort of resin. Although hemoperfusion may be more effective than hemodialysis in clearing the blood of many protein-bound drugs (because the charcoal or resin in the cartridge will compete with plasma proteins for the drug, adsorb the drug, and thereby remove it from the circulation), modern high-flux dialyzers may also perform in a similar manner. Hemoperfusion will remove many lipid-soluble drugs from the blood much more efficiently than hemodialysis. In the United States, hemoperfusion cartridges are expensive and have been discontinued by some manufacturers, and with a short shelf life of 2 years, may not be available in certain urban cities (Shalkam, 2006). If a drug is equally well removed from the blood by hemoperfusion and hemodialysis, then hemodialysis is preferred: potential problems of cartridge saturation are avoided, and the incidence of hemoperfusion complications such as thrombocytopenia and leukopenia is reduced; plus, with hemodialysis, any coexisting acid–base or electrolyte disturbances can be treated.
4. Continuous hemodiafiltration, hemoperfusion. Prolonged continuous treatment is potentially useful in drugs with moderately large volumes of distribution (VD) and slow intercompartmental transfer times, because posttherapy rebound of plasma drug levels is avoided. Clear advantages of continuous treatment over repeated conventional treatments for drug rebound remain to be demonstrated. Continuous hemoperfusion has been used successfully in theophylline and phenobarbital toxicity, and continuous hemodiafiltration has been used in ethylene glycol and lithium toxicity (Leblanc, 1996).
C. Toxicokinetics. Poisons have various molecular characteristics that make them more or less amenable to extracorporeal removal. Dialyzability of a poison is possible only if it can be extracted from the plasma compartment, if a significant proportion of its total body stores can be eliminated, and if extracorporeal clearance contributes a significant amount to total clearance. Removal from the plasma compartment is best reflected by the dialyzer extraction ratio, which can be calculated as (A-V)/A, where A represents the inflow (prefilter or precolumn) concentration of the solute to be removed, and V represents the dialyzer outflow concentration. The amount of toxin that can be removed by extracorporeal treatment is greatly affected by the volume of its distribution in the body. The ratio of extracorporeal removal to endogenous removal depends on the endogenous clearance of a particular poison and the current condition of the body organs (liver and/or kidney) that normally participate in endogenous removal. The following factors will influence poison dialyzability (Lavergne, 2012):
1. Molecular weight. Extracorporeal modalities have different molecular weight cutoffs; techniques that use diffusion such as hemodialysis usually have an approximate cutoff of 5,000 Da, while convection- and adsorption-based techniques are capable of removing poisons that are in excess of 50,000 Da in size. Plasmapheresis can remove poisons that are up to 1,000,000 Da in size.
2. Protein binding. Since the poison–protein complex cannot freely pass through dialyzers or hemofilters, only poisons that are largely unbound (or free) can be removed by these techniques. However, at higher concentration (such as in overdose), protein binding of a drug can become saturated; under such conditions, a higher proportion of the drug is unbound or “free,” which is then available for removal by extracorporeal treatment.
3. Volume of distribution. VD is the theoretical volume into which a drug is distributed. Heparin, for example, a drug confined to the blood compartment, has a VD of approximately 0.06 L/kg. Drugs distributed primarily in the extracellular water (e.g., salicylates) will have a VD of approximately 0.2 L/kg. Some drugs will have VD values exceeding the volume of total body water because they are extensively bound to, or stored in, tissue sites. With drugs that have a high VD (e.g., digoxin, tricyclics), the amount of drug present in the blood represents only a small fraction of the total body load. Thus, even if a hemodialysis or hemoperfusion treatment extracts most of the drug present in the blood flowing through the extracorporeal circuit, the amount of drug removed during a single treatment session will represent only a small percentage of the total body drug burden. Subsequently, additional drug will enter the blood from tissue stores, sometimes causing a recurrence of the toxic manifestations. On the other hand, even transiently lowering the blood concentration of many drugs may mitigate certain important toxic effects of these agents. Hence, hemodialysis or hemoperfusion can sometimes effectively reduce drug toxicity even when the VD is large.
4. Endogenous clearance. Extracorporeal removal usually is not indicated when endogenous clearance by metabolism and elimination is expected to exceed the rate of exogenous elimination. This explains why hemodialysis is not indicated for poisons like cocaine or toluene. Similarly, the presence of kidney impairment for renally eliminated poisons (e.g., lithium) will make extracorporeal removal more important.
D. Technical points
1. Vascular access for hemodialysis or hemoperfusion in poisoning. In patients without permanent vascular access in place, percutaneous cannulation of a large central vein using a dialysis catheter is required.
2. Choice of hemodialyzer. High-flux, high-efficiency dialyzers with high urea clearances should generally be used. The development of high-cutoff hemodialysis membranes (with increased pore size of 8 to 10 nm) may allow clearance of larger toxins and molecules as large as 50 to 60 kDa (e.g., Fab fragments).
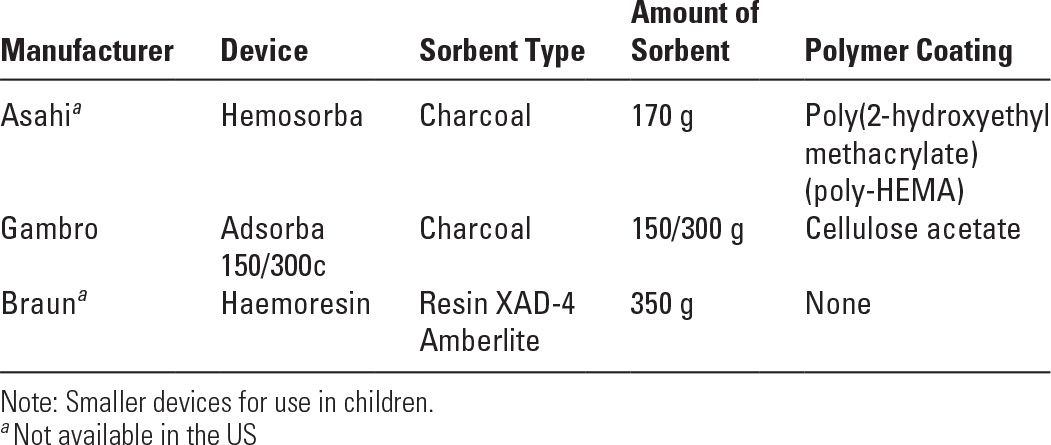
3. Choice of a hemoperfusion cartridge. Some of the available cartridges are listed in Table 20.3. Typical sorbents are activated carbons (charcoals), ion exchange resins, or nonionic exchange macroporous resins. Sorbent particles have been rendered biocompatible by coating the surface with a polymer membrane. The cartridges contain various amounts of sorbent, the smaller ones being designed for pediatric use. A detailed comparative evaluation of in vivo performance of the various brands of cartridges has been published (Ghannoum, 2014).
4. The hemoperfusion circuit. The hemoperfusion circuit is similar to the blood side of a hemodialysis circuit and includes an air detector and a venous air trap. Standard hemodialysis blood pumps and machines (without use of dialysis solution) are often used to drive the blood through the tubing and cartridge.
5. Priming the hemoperfusion circuit. Setup and priming with saline or dextrose differ depending on the brand of cartridge used, and the manufacturer’s literature should be consulted in all instances. The hemoperfusion cartridge must be primed in a vertical position with the arterial (blood inlet) side facing downward.
6. Heparinization during hemoperfusion. Once the cartridge has been primed, a bolus dose of heparin (usually 2,000–3,000 units) is administered into the arterial line, the cartridge is kept inlet side down, and blood flow through the cartridge is begun. As a rule, because of some adsorption on the sorbent, more heparin may be required for a hemoperfusion treatment (e.g., approximately 6,000 units or 10,000 units for charcoal and resin, respectively, per session) than for hemodialysis. Heparin should be given in amounts sufficient to maintain the patient’s activated clotting time (ACT) or partial thromboplastin time at about twice the normal value.
Some Available Hemoperfusion Devices (May Vary by Country) | |
7. Duration of hemoperfusion. A single 3-hour treatment will substantially lower the blood levels of most poisons for which hemoperfusion is effective. More prolonged use of a hemoperfusion cartridge is inefficient, because the charcoal tends to become saturated. Replacement of saturated devices with fresh ones is not usually required, and any rebound in blood drug concentrations consequent to tissue release can be treated with a second hemoperfusion session. On the other hand, a continuous hemoperfusion treatment may need to be prolonged for several days until clinical improvement or a nontoxic blood level is achieved. Hemoperfusion devices may need to be changed every 4 hours in the course of continuous treatment.
E. Complications. All extracorporeal techniques require vascular access through a central line, and this procedure itself is subject to complications.
1. Hemodialysis
a. Hypophosphatemia. In contrast to end-stage renal disease (ESRD), patients being dialyzed for poisoning often do not have an elevated plasma phosphate value. Because phosphate is not present in standard dialysis solutions, intensive dialysis can severely lower the plasma phosphate level, resulting in respiratory insufficiency and other complications. Hypophosphatemia during dialysis can be avoided by supplementing the dialysis solution with phosphate as discussed in Chapter 10.
b. Alkalemia. Standard hemodialysis solutions contain unphysiologically high concentrations of bicarbonate and they also contain bicarbonate-generating base in the form of acetate or citrate, as they have been designed to correct metabolic acidosis. Performing dialysis for poisoning in a patient with metabolic or respiratory alkalosis can provoke or worsen alkalemia unless the dialysis solution bicarbonate concentration is appropriately reduced.
c. Disequilibrium syndrome in acutely uremic patients. In patients with both severe uremia and poisoning, it may be dangerous to carry out a prolonged high-clearance dialysis session initially. During a dialysis treatment for a metformin-associated lactic acidosis in a markedly uremic patient, enrichment of dialysate with an appropriate amount of urea in an attempt to attenuate the manifestations of the disequilibrium syndrome has been successfully performed (Doorenbos, 2001).
2. Hemoperfusion. Mild transient thrombocytopenia and leukopenia can occur, but cell counts usually return to normal within 24 to 48 hours following a single hemoperfusion. Adsorption or activation of coagulation factors has also been observed rarely and may be clinically significant in patients with liver failure.
3. Continuous therapy. Fluid and electrolyte imbalances may be potential problems and require frequent monitoring. Prolonged anticoagulation may predispose to bleeding.
II. MANAGEMENT OF POISONING WITH SELECTED AGENTS
A. Acetaminophen (MW 151 Da). Activated charcoal should be given to patients presenting within 4 hours of ingestion. Serum levels should be measured and plotted using the Rumack–Matthew nomogram to establish the risk of hepatotoxicity and need for N-acetylcysteine (NAC) therapy. Concomitant ingestion of moderate amounts of ethanol markedly increases the risk of liver damage. If serum acetaminophen levels are above 150 mg/L (1.0 mmol/L) at 4 hours, the likelihood of toxicity is high and NAC (PO or IV) should be given. NAC, by increasing reduced glutathione stores, prevents the accumulation of toxic acetaminophen by-products. Its efficacy in preventing liver failure declines if started more than 10 hours after ingestion, but NAC is still recommended even after 24 hours. Although acetaminophen is moderately water-soluble and is minimally protein-bound and thus removed by dialysis or hemoperfusion, NAC remains the treatment of choice.
B. Aspirin (acetylsalicylic acid, MW 180 Da). In adults, severe aspirin poisoning is usually accompanied by metabolic acidosis with respiratory alkalosis, whereas in children, isolated metabolic acidosis is often encountered. The appearance of central nervous system (CNS) symptoms is a sign of severe poisoning. The Done nomogram (Done and Temple, 1971), relating serum levels and time of ingestion to outcome, gives some idea of the seriousness of salicylate poisoning in children, but is less used in adult poisoning. MDAC should be initiated and urine alkalinization carried out if substantial urine output is achievable, particularly when symptoms are present and serum salicylate levels are >50 mg/dL (2.8 mmol/L). Aspirin has a VD of only 0.15 L/kg. Despite the fact that the drug is about 50% protein-bound, aspirin is well removed by hemodialysis. Hemodialysis should be considered when the serum level exceeds 90 mg/dL (6.5 mmol/L) or there is evidence of marked acidemia, neurologic involvement (neurologic symptoms, hyperthermia, seizures) or noncardiogenic pulmonary edema.
C. Barbiturates. Toxic serum levels of phenobarbital (MW 232 Da) are over 3 mg/dL (130 mcmol/L), and coma begins to appear at levels of 6 mg/dL (260 mcmol/L). MDAC should be considered as first-line therapy, and alkalinization of the urine may help remove long-acting barbiturates such as phenobarbital. Phenobarbital is 50% protein-bound, but its VD is only 0.5 L/kg; the drug is well removed by either hemodialysis or hemoperfusion. Hemodialysis should be contemplated when coma is prolonged, especially when complications of coma, such as pneumonia, threaten. Removal with hemodialysis using a synthetic membrane dialyzer equals that of hemoperfusion (Palmer, 2000).
D. Digoxin (MW 781 Da). The probabilities of digoxin-induced arrhythmias are 50% and 90% at serum levels of 2.5 and 3.3 ng/mL (3.2 and 4.2 nmol/L), respectively. Treatment includes correction of hypokalemia, hypomagnesemia, and alkalosis and administration of oral-activated charcoal.
The VD of digoxin is large (8 L/kg in normal patients, 4.2 L/kg in dialysis patients), and the drug is 25% protein-bound. For these reasons, only 5% of the body load will be removed by a 4-hour hemodialysis treatment. Although hemoperfusion is more effective and has been shown to improve symptoms, it is not routinely recommended in the treatment of digoxin toxicity as the VD of the drug is so large that total body clearance is limited. Plasmapheresis performed soon after Fab fragment administration promotes removal of the Fab–digoxin complexes (Zdunek, 2000), and high-cutoff membranes such as Theralite can be used for this purpose also (Fleig, 2011). Most authors recommend an additional Fab treatment if toxicity recurs. In dialysis patients, Fab therapy remains preferred over hemoperfusion or plasmapheresis. Although Fab has been used successfully in patients with coexisting renal failure, digoxin may be released from the Fab–digoxin complex, leading to a rebound in toxicity, perhaps requiring a second treatment (Ujhelyi, 1993).
E. Toxic alcohols.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





