Fig. 2.1
Fractions of circulating total testosterone
SHBG can vary considerably in men, greatly affecting total testosterone levels. Because SHBG-bound testosterone is not bioavailable, relying solely on total testosterone as an indicator of the adequacy of circulating androgens available for physiologic effect may lead to failure of diagnosing hypogonadism. Common medical conditions that increase SHBG include aging, hyperthyroidism, and hepatic cirrhosis. Those that decrease SHBG include obesity, diabetes mellitus, and glucocorticoid use. These are summarized in Table 2.1. Variability in SHBG levels makes the consideration of free and bioavailable testosterone important, in order to better appreciate the physiologically active circulating testosterone concentration. For example, the increase in SHBG with age means that older men may demonstrate a normal total testosterone level, even if they are hypogonadal, with low levels of free or bioavailable testosterone. Conversely, obesity decreases SHBG and total testosterone, even when the bioavailable fraction may be normal [4].
Table 2.1
Factors affecting SHBG levels
Factors that increase SHBG | Factors that decrease SHBG |
|---|---|
Aging | Obesity |
Cirrhosis | Nephrotic syndrome |
Hepatitis | Acromegaly |
Hyperthyroidism | Hypothyroidism |
Anticonvulsants | Glucocorticoids |
Estrogens | Progestins |
HIV disease | Androgenic steroids |
Diabetes mellitus |
It should be noted that independent of SHBG levels, many of the features of the metabolic syndrome, such as hypertension, dyslipidemia, insulin resistance, and obesity, are commonly present in hypogonadal men [22, 23]. Hypogonadotropic hypogonadism occurs frequently in men with type II diabetes mellitus [24]. Expert opinion encourages the measurement of testosterone in men with metabolic syndrome and symptoms of testosterone deficiency, but the utility of androgen replacement therapy in these men continues to be an active area of discussion and research [3].
Laboratory Measurement of Testosterone
Testosterone assays and their interpretation pose several challenges. Testosterone concentrations in plasma vary more than three orders of magnitude depending on age, gender, and the presence of disease, and an adequate assay must be able to maintain sensitivity and specificity over this large range of concentrations. Other steroids in the circulation that are of similar structure and concentration as testosterone can lead to difficulties with assay interpretation and falsify results. Age, ethnicity, and gender-adjusted normal ranges, using a standardized assay, are lacking. Furthermore, there is no universally recognized testosterone-calibration standard [5].
The majority of circulating testosterone is bound to carrier proteins, which also has implications for accurate measurement of testosterone concentration. Early testosterone assays required testosterone to be extracted or displaced from SHBG and albumin by dissolution into organic solvents, separated by chromatography, and then measured (Fig. 2.2). This method offered several advantages, including the separation of interfering proteins and cross-reacting steroids to increase specificity, and the use of large serum aliquots to increase sensitivity. Unfortunately, this method was also time-intensive and expensive, and typically limited to research laboratories. With increased demand for economical and rapid alternatives, these early assays were replaced by radioimmunoassays (RIAs), which are presently the longest-used tests for the measurement of serum testosterone. Most of the current “normal ranges” are based on RIA results.
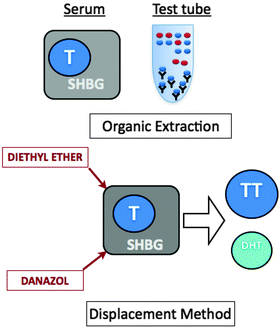

Fig. 2.2
Testosterone extraction and displacement
Over the last decade, the laboratory investigation of total testosterone measurement has evolved from RIA testing after serum extraction, to direct automated enzyme-linked immunoassay (ELI) testing available in most laboratories, to mass spectroscopy methods in reference laboratories. Each approach has its advantages and disadvantages. Correlation between these various methodologies can be poor, and national standardization is necessary in this area for portability and comparability of results obtained from the same patient.
Radioimmunoassay
RIAs are based on competitive binding of testosterone to a testosterone-specific antibody. The patient’s serum is mixed with a set amount of radioactively labeled testosterone tracer and a fixed amount of antibody against testosterone. The amount of tracer displaced by the patient’s testosterone is evaluated by measuring the radioactivity of the sample, and the patient’s testosterone concentration is calculated. This method is illustrated in Fig. 2.3.
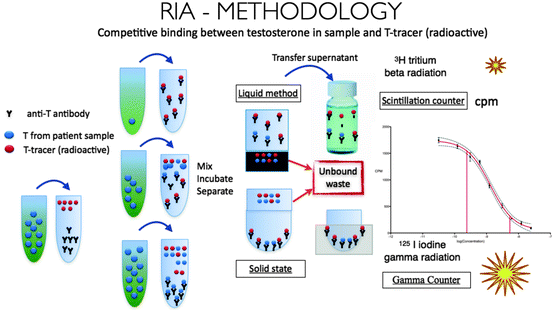

Fig. 2.3
Radioimmunoassay for measurement of serum testosterone
Traditional RIAs involve a testosterone extraction step, which increases their sensitivity and accuracy. These assays exhibit a variability of less than 10% in an experienced laboratory, with a lower limit of detection less than 10 pg [25]. Unfortunately, they require a technically experienced staff, are time-intensive, and generate large amounts of radioactive waste. Commercially available RIA kits are an easier alternative. However, they use a set amount of tracer and antibody, with the result that their ability to measure very high or very low levels of testosterone is somewhat poor. The detection of low levels of testosterone can be improved by increasing the amount of antibody while reducing the amount of tracer [4]. This concept is illustrated in Fig. 2.4, which shows an immunoassay calibration curve. The testosterone concentration of a sample is accurately calculated over the dynamic range, represented by the linear portion of the curve. The sensitivity and specificity of the assay is decreased at low testosterone concentrations, where the curve is no longer linear. Changes in the amount of antibody and/or tracer in the assay alter the binding capacity and shifts the curve, thereby affecting the dynamic range (Fig. 2.4).
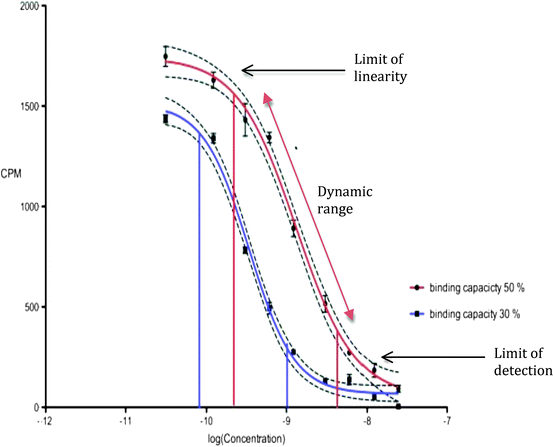

Fig. 2.4
Calibration curves for testosterone assays
The RIA requires a specific antibody with minimal cross-reactivity. Testosterone, like most steroids, is a poor antigen. Therefore, the design of a testosterone-specific antibody is, therefore, difficult. For commercially available kits, each manufacturer usually uses a different antibody. This may in turn cause a different binding affinity to testosterone, as well as different cross-reactivity. These differences can certainly contribute to the variable results seen with different commercial kits.
Enzyme-Linked Immunoassay
Like RIAs, EIAs are also based on the principle of competitive binding to an antibody. Instead of being radioactively labeled, the tracers used in EIA are fluorescent or chemiluminescent, which eliminates the possibility of radioactive waste. EIAs are easy to automate, and are widely used in non-reference hospital and commercial laboratories.
EIAs are subject to most of the same shortcomings of RIAs, namely, limited utility at very low or very high testosterone concentrations, and the need for a testosterone-specific antibody, which can vary by manufacturer. While these automated assays are sufficient for most clinical purposes in men, results can vary by as much as 33% among different assay methods. Even values in normal men can fall out of the manufacturer’s reference range [14]. Additionally, EIAs do not routinely include a testosterone extraction step. Therefore, they do not necessarily measure true total testosterone.
Some manufacturers of commercial RIA and EIA use danazol for testosterone displacement as an alternative to testosterone extraction using organic solvents. In theory, this additional step is designed to improve the sensitivity and accuracy of the assays. However, the amount of danazol present in the kits is fixed and limited, and usually insufficient in displacing testosterone from samples with elevated amounts of SHBG.
Liquid Chromatography-Tandem Mass Spectroscopy
Direct methods of measuring testosterone have gained popularity over the last decade, due to the inconsistencies experienced with indirect immunoassays. Reference laboratories are increasingly using liquid chromatography-tandem mass spectroscopy (LC-MS), which combines automation and high-throughput with high precision, reproducibility, and wide linearity of assay. Steroid hormones are extracted from the serum sample by the use of organic solvents and separated by liquid chromatography, and testosterone levels are then determined by peak area integration of testosterone-containing fractions of the column eluate (Fig. 2.5). LC-MS has a lower limit of detection of 15 ng/dL, with an intra-assay coefficient of variation of <10% [14]. The specificity of this assay is enhanced by a distinct mass spectroscopy fingerprint for all known steroids, from which testosterone can be specifically selected. LC-MS is recognized as the most reliable method of measuring total testosterone in women, children, and hypogonadal men [26, 27], and has been accepted as the new gold standard assay for testosterone measurement.
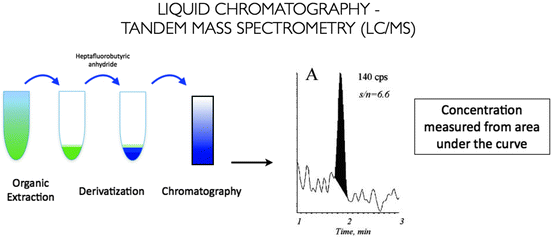

Fig. 2.5
Liquid chromatography-mass spectroscopy for measurement of serum testosterone
Laboratory Measurement of Testosterone Fractions
Assays for various testosterone fractions have also been developed, and are summarized in Table 2.2. Some laboratories have directly measured bioavailable testosterone by selective ammonium sulfate precipitation of SHBG. Others have measured free testosterone by equilibrium dialysis, and advocated for this being the most accurate assessment of physiologically active circulating hormone fraction. The calculation of free testosterone, bioavailable testosterone, and the free androgen index (FAI = testosterone/SHBG) is described as an indirect means of assessing testosterone concentration.
Table 2.2
Assays for the measurement of testosterone, free testosterone, and bioavailable testosterone
Total testosterone | Radioimmunoassay (RIA) |
Enzyme-linked immunoassay (EIA) | |
Liquid chromatography-mass spectroscopy (LC-MS) | |
Free testosterone | Equilibrium dialysis |
Ultracentrifugation | |
Radioimmunoassay (RIA) | |
Calculated free testosterone | |
Bioavailable testosterone | Ammonium sulfate precipitation of SHBG |
Calculated bioavailable T |
According to the “free hormone hypothesis,” only free testosterone is available to the end organs and biologically active at the tissue level [28]. However, recent studies have suggested that even SHBG-bound testosterone can be internalized into cells via endocytosis, questioning the validity of the free hormone hypothesis. Because of these conceptual uncertainties, while some authors continue to argue in favor of using free testosterone assays or calculations, expert opinion at this time favors the use of total testosterone levels for the diagnosis of androgen deficiency.
Measurement of Free Testosterone
Unfortunately, there is no cost-effective, simple, widely available, reliable, or accurate method of directly measuring free testosterone. The serum concentration of free testosterone is usually 50–100 times lower than the concentration of total testosterone; therefore, lower limits of detection are important to consider for any assay measuring free T. The free hormone fraction also needs to be separated from bound testosterone in order to be measured.
Equilibrium dialysis is the gold standard assay for the measurement of free testosterone. The patient’s serum is placed in a dialysis chamber with two compartments. One compartment contains 3 H-T (tracer-labeled testosterone) added to the serum, while the other contains a buffer solution. The compartments are separated by a semipermeable membrane with a low molecular weight cut-off. Dialysis is performed until testosterone concentrations within the two compartments reach an equilibrium value. Free and bound 3 H-T are then separated and measured. This value is multiplied by the total testosterone concentration. Although highly precise, equilibrium dialysis methods are expensive and complex, with potential errors due to temperature, sample dilution, and tracer impurities [14]. For example, a 2% contamination, with a tracer that does not bind to proteins can cause a doubling of the free testosterone concentration [5]. The value and accuracy of total testosterone has a bearing on the free testosterone concentration obtained with equilibrium dialysis.
One alternative to equilibrium dialysis for the direct measurement of free T is ultracentrifugation, a process where free T is separated from bound testosterone using ultrafast centrifuges, and then directly measured [29]. The direct measurement of free testosterone by RIA has also been described, but does not provide an accurate assessment when there are alterations in SHBG [30, 31]. The use of this technique is not recommended [32].
Calculation of Free Testosterone
Free testosterone can be calculated based on the concentration of SHBG, albumin, and total testosterone. Several different calculations for free T have been described in the literature [33]. These equations require the use of equilibrium dissociation constants for the binding of SHBG and albumin to testosterone, which are usually defined as 1 × 109 and 3 × 104 L/M, respectively. These numbers are not universally agreed upon, which can lead to discrepant results when the equations are compared [33]. The albumin concentration in serum is assumed to be 4.3 g/dL for the purposes of this calculation; changes in the albumin level within normal physiologic range have been shown to have little impact on the calculated free testosterone [30]. However, because the calculated value of free T depends on measured SHBG and total testosterone levels, measurement error and variable reference ranges for these two analytes directly impact the results. With the exception of certain metabolic conditions such as obesity, hyperthyroidism, and low cortisol, calculated free T correlates very well with free T obtained by equilibrium dialysis [30]. In contrast, studies comparing calculated values with those obtained by ultrafiltration do not always show high degree of correlation [34].
Free Androgen Index
The FAI represents the ratio of total testosterone and SHBG: testosterone/SHBG. The ratio is easy to calculate, and valid in serum samples from women. There is a reasonable correlation between FAI and FT, but it is not consistently maintained at low testosterone levels [35]. FAI is less useful in men because the majority of SHBG in men is bound to testosterone. Like the calculated free testosterone, the FAI is also dependent on accurate values for testosterone and SHBG.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





