Neuroendocrine tumors are increasingly diagnosed, either incidentally as part of screening processes, or for symptoms, which have commonly been mistaken for other disorders initially. The diagnostic workup to characterize tumor behaviour and prognosis focuses on histologic, anatomic, and functional imaging assessments. Several therapeutic options exist for patients ranging from curative and debulking surgery through to liver-directed therapies and systemic treatments. Multimodal therapies are often required over the patient’s disease history. The management paradigm can be complex but should be focused on curative resections and then on controlling symptoms and limiting disease progression. There are several new systemic therapies that have completed phase 3 studies with new compounds being studied in phase 2. Genetic and epigenetic markers may lead to a new era of personalised therapy in the future.
Key points
- •
Neuroendocrine tumors are heterogenous in nature and increasing in incidence.
- •
Symptoms can develop from secreted bioactive substances or from the mass effect of the tumor.
- •
Anatomic and functional imaging modalities are helpful in staging disease and assessing tumor biology.
- •
A range of therapies is available that include surgical, liver directed, and systemic therapies.
- •
The treatment of neuroendocrine tumors can be multimodal over a patient’s disease history.
Introduction
Neuroendocrine tumors (NETs) of the gastrointestinal tract arise at many different sites, including the pancreas and small bowel, and are heterogeneous in nature with increasing incidence. This review of gastroenteropancreatic (GEP) NETs discusses epidemiology, presentation, diagnosis, and management of primary and secondary sites of disease. Gastroenterologists have traditionally diagnosed and managed these tumors but now have a core role in multidisciplinary tumor board meetings on therapy decisions like surgery and systemic therapies. Significant advances have been made in managing these tumors with new diagnostics techniques and therapies. Many patients are now informed about their condition with information from Web sites and patient support groups with an expectation to access the whole array of diagnostic and therapeutic modalities.
NETs originate from neuroendocrine cells in the pancreatic islet and gastroenteric tissue. Small bowel (sb) and pancreatic (p) NETs have different clinical and genetic signatures and were previously considered to be largely benign in nature. The World Health Organization (WHO) 2010 nomenclature considers all NETs as malignant and classifies them by the cellular proliferation and degree of differentiation. The use of NET rather than the historical “carcinoid” tumor is preferred and encouraged for describing gastrointestinal and pNETs. Similarly, classification by primary tumor site rather than embryologic origin (foregut/midgut/hindgut) is the accepted nomenclature. The molecular biology of NETs is still poorly understood, but there are emerging data to suggest that profiling of genetic and molecular signatures may enhance tumor classification and identify potential targets that may be involved in tumor progression ( Table 1 ).
| WHO (2010) and ENETS Nomenclature | Grade | Mitotic Count | Ki-67 Index (%) | Cell Type |
|---|---|---|---|---|
| NET | G1 | <2 mitoses/10 HPF | ≤2 | — |
| NET | G2 | 2–20 mitoses/10 HPF | 3–20 | — |
| Neuroendocrine carcinoma (NEC) | G3 | >20 mitoses/10 HPF | >20 | Large vs small cell |
Epidemiology
A key problem with NET epidemiology has been changes to classification systems and reliance on registries. International Classification of Diseases for Oncology-10 uses histopathological coding and has been used in most recent registries. It includes all the tumors that were previously classified as benign, which may be partly contributing to the increased incidence since 2000. The largest registry is the Surveillance, Epidemiology, and End Results database that spans more than 5 decades and 15% of the US population from specific states. There are national population studies that contribute to defining incidence in the United Kingdom, Norway, Sweden, Ireland, Netherlands, Denmark, and Austria. NETs may now be the most common small bowel tumor (37.4%), ahead of adenocarcinoma (36.9%), lymphomas (17.3%), and stromal tumors (8.4%).
There are reported ethnic differences with African Americans having the highest NET incidence at 6.5 per 100,000 persons. The overall incidence of NETs in Caucasians in the United States and Norway is 4.44 and 3.24 per 100,000 persons, respectively. The rectum is the commonest site in the United States and Far East, with lung NETs the commonest site in Caucasian US patients. The incidence of NETS of the appendix, cecum, and pancreas almost doubled between 1975 and 2005, but these tumors are only a fraction of NETs diagnosed, around 0.1 to 0.2 cases per 100,000 persons. Historical autopsy studies in Sweden described an incidence of 8.4 per 100,000 with a significant number of NET tumors that were not diagnosed antemortem. The prevalence of NET is proportionally much greater than the incidence because of improved survival when compared with other common cancers like gastric and pancreatic adenocarcinomas. Whatever the precise incidence of NETs, it appears that the number of patients presenting with these tumors has been steadily increasing.
Genetics
GEP-NETs may be associated with familial endocrine cancer syndromes, such as pNETs with multiple endocrine neoplasia type 1 (MEN1) and less commonly with von Hippel-Lindau and tuberous sclerosis. The incidence of MEN1 in GEP NETs varies from rare in gastrointestinal (GI) NET, to 5% in insulinomas, and 25% to 30% in gastrinomas. The diagnosis of MEN1 can now be confirmed by testing for the presence of the MENIN gene mutation. Mutations involving the succinate dehydrogenase subunit D, usually associated with paragangliomas and pheochromocytomas can be associated with sbNETs.
There are sb and colon “NET families” (non-MEN1) described in which more than one family member has been diagnosed with an NET. Standardized incidence rates of 4.35 for small intestinal and 4.65 for colon NETs occur in offspring of parents affected by NETs. Candidate genes for these findings have been proposed.
There has been recent interest in somatic mutations occurring in these tumors. Jiao and colleagues sequenced tissue from pNET and found an excess of mutations in the menin, DAXX, ATRXX, and mTOR genes. Differences in survival related to the presence of these genes have been debated, but the work may lead to personalized medicine based on genetics results.
Clinical presentations of gastroenteropancreatic neuroendocrine tumors
NETs are described in the pancreas and all sites of the gastrointestinal tract. The behavior of GEP-NETs does differ by primary site with symptoms varying from the incidental diagnosis through to obstructive mass effect and symptomatic syndromes from the secretion of bioactive agents. There may be a significant delay between onset of symptoms and diagnosis. Diarrhea and flushing are known specific symptoms, but frequently fatigue is the worst symptom with depression also common. Quality-of-life (QoL) research in GEP-NETs is a comparatively new field, with a disease-specific QoL questionnaire, the QLQ-GINET21, and the Norfolk NET questionnaire being used in trials for GI NET. Symptomatic patients frequently have liver metastases at diagnosis. The liver lesions are highly vascular and can be “functionally” active secreting vasoactive substances or hormones that can cause systemic symptoms. The 4 commonest GEP-NET primary sites are discussed later ( Table 2 ).
| Type of NET | No. | Mean Age at Diagnosis | Mean Duration 1st Symptom (Range, mo) | Mean Age at 1st Symptom (y) | Age Over 50 at 1st Symptom (%) |
|---|---|---|---|---|---|
| Appendix | 14 | 44.2 | 46.8 (2–180) | 41.7 | 29 |
| Lung | 51 | 50.7 | 67.7 (1.5–360) | 46.2 | 54 |
| Pancreas | 64 | 49.2 | 39.1 (0–240) | 46.6 | 42 |
| Small bowel | 99 | 55.2 | 60.1 (0–300) | 50.8 | 69 |
| Stomach/gastric | 14 | 55.1 | 38.5 (1–144) | 53.0 | 71 |
| Unknown Primary | 33 | 52.9 | 43.4 (1.5–204) | 50.4 | 55 |
| Grand total | 275 | 52.1 | 53.3 (0–360) | 48.6 | 56 |
Small Bowel
sbNETs are equal to rectal tumors in being the next most common primary site of all NETs (after lung) with an incidence of 17.2%. The most common site is the last 60 cm of distal ileum with a quarter being multifocal tumors. Common symptoms are of abdominal pain as well as intermitted bowel obstruction that can result from the mechanical effect of the primary tumor, or from mesenteric lymph node involvement, secondary desmoplasia, and bowel ischemia from vessel involvement. sbNETs can cause the classical carcinoid syndrome of diarrhea, flushing, palpitations, and bronchospasm, which develop in the context of serotonin-secreting liver metastases. Carcinoid heart disease can develop from fibrosis of the tricuspid and pulmonary valves, leading to right heart failure in patients with carcinoid syndrome and elevated serotonin. Prolonged serotonin production in carcinoid syndrome may lead to nicotinamide deficiency, causing lacrimation, rhinorrhea, and diarrhea.
Rectum
The incidence of rectal NETs has increased rapidly to 17% of all NETs and now equals that of sbNETs in recent years. Most patients are asymptomatic with over half of all rectal NETs diagnosed incidentally at endoscopy. Rectal NETs have the best overall survival of GEP-NETs, with almost 90% less than 1 cm and localized to the submucosal.
Stomach
The incidence of gastric NETs has increased to 6.0% of all NETs between 2000 and 2007. Gastric NETs are invariably diagnosed at endoscopy and arise from enterochromaffin-like cells involved in the regulation of gastric acid production. Three subtypes are described with type 1 and type 2 developing in the presence of hypergastrinemia, and type 3 occurring independently of gastrin. Type 1 gastric NETs develop from secondary hypergastrinemia stimulation from achlorhydria environments like chronic atrophic gastritis. Type 2 gastric NETs develop from autonomous hypergastrinemia stimulation from a gastrinoma that can cause marked acid secretion and peptic ulceration. Histamine secretion can occur in gastric NETs causing allergic-type symptoms.
Pancreas
The incidence of pNETs is approximately 6% of all NETs, with a less rapid increase when compared with rectal and gastric NETs incidences that are more directly influenced by increased endoscopy. Almost half of pNETs are functional with symptoms that result from bioactive secretion as outlined in later discussion. The value of classifying tumors by specific hormone output has been challenged, although it is still common practice. Nonfunctional pNETs are often incidental findings on cross-sectional imaging, but a proportion of patients present with symptoms from mass effect, such as biliary obstruction, or from metastatic disease ( Table 3 ).
| Tumor Type | Symptoms |
|---|---|
| Insulinoma | Dizziness, irritability, sweating, fits, coma, response to food |
| Gastrinoma | Small bowel perforation, duodenal ulceration with bleeding, diarrhea responding to PPI therapy |
| Glucagonoma | Diabetes, migratory rash, diarrhea, stomatitis |
| VIPoma | Severe diarrhea, weight loss, hypokalemia |
| Somatostinoma | Gall stones, weight loss, diarrhea, steatorrhea, diabetes |
| PPoma | Usually no specific symptoms; weight gain and constipation can occur |
Diagnostic modalities
Anatomic imaging modalities, such as computed tomography (CT) and MRI, characterize the extent of NET disease to assist with staging and planning therapy. In particular, CT provides an anatomic map for both curative and debulking cytoreductive surgical resections. Functional imaging modalities, like octreotide scintigraphy and PET-CT, provide evidence of biological behavior that, as discussed later in the review, indicate the role for the specific medical therapies in disease management.
Anatomic Imaging
CT is the dominant anatomic imaging modality for NETs, which exhibit avid early enhancement (pNETs in particular) on biphasic or triphasic contrast-enhanced CT. Unenhanced scans can demonstrate an isodense lesion with calcification in 20% of pNETs in contrast to the uncalcified appearances of pancreatic adenocarcinomas. pNETs typically show homogenous avid arterial enhancement with contrast. Nonfunctional pNETs are typically larger than functional pNETs and can be heterogenous lesions with necrosis and cystic degeneration as well as can cause mass effect on surrounding structures like the biliary system.
sbNETs typically cause a marked desmoplastic reaction in the mesentery with fat changes, tethering, and stranding that are easily discernible on CT. In addition, nodal metastases to the root of the mesentery can encase vessels like the superior mesenteric vein (SMV) and superior mesenteric artery (SMA) with consequent radiological signs of bowel ischemia. Metastatic disease to the liver enhances in a similar pattern to the primary NET. The hepatic arterial phase is useful in identifying liver metastases. The sensitivity of CT in detecting primary, regional, and metastatic disease increases with lesion size.
MRI is a useful imaging modality in NETs given the burden of ionizing radiation exposure from diagnosis through to surveillance in patients. pNETs typically exhibit T2-weighted (T2W) hyperintensity and T1-weighted (T1W) hypointensity with moderate but diffuse contrast enhancement. Two-thirds of sbNETs can be identified on MRI and are more easily discernible on postgadolinium contrast T1W fat-suppressed images. MRI can help characterize liver metastases when CT is equivocal or when the background liver is steatotic. Lesions are more easily identified on T2W and hepatic arterial phase T1W fat-suppressed imaging. Diffusion-weighted imaging is useful in characterizing NETs, particularly pNETs and hepatic metastases, as well as assessing response to liver directed therapy ( Figs. 1 and 2 ).
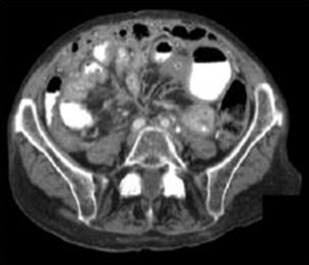
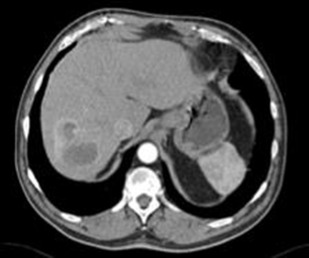
Functional Imaging
Somatostatin receptors (SSTR) are differentially expressed in NETs with most GEP-NETs expressing the subtype SSTR2. Functional imaging modalities assess for SSTR2 expression and can provide additional staging and prognostic information beyond cross-sectional modalities. Importantly, patients with lesions that are SSTR2-avid can benefit from specific therapeutic options that are discussed later. 111 Indium-octreotide scintigraphy (OctreoScan) and PET-CT with 68 Ga-DOTATATE are useful modalities for assessing for avid disease in patients with low-grade (G1 or G2) and well-differentiated GEP-NETs. 68 Ga-DOTATATE PET has a higher sensitivity and specificity than 111 Indium-octreotide scintigraphy. High-grade (G3) and poorly differentiated NETs have lower expression of SSTR and are consequently poorly avid. However, these tumor lesions are metabolically more active and take up the glucose analogue on PET with fludeoxyglucose F 18 imaging ( Figs. 3 and 4 ).
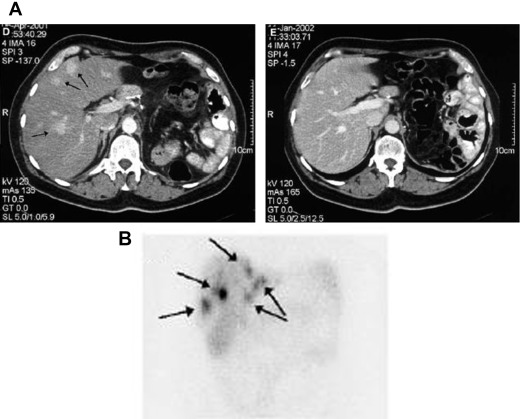
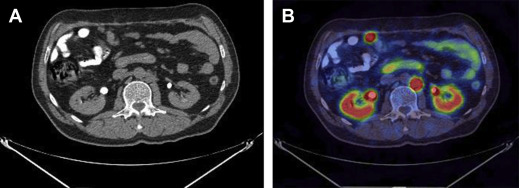
Markers of neuroendocrine tumors disease
Circulating and tissue markers of NET disease can be used to assess for disease, prognosis, treatment response, and recurrence. Existing markers may be general, like chromogranin A (CgA) and serotonin, or specific to subtypes of NETs like gastrin and insulin. More novel markers, like mRNA transcript panels and circulating tumor cells, are likely to have a role in the future.
Chromogranin A
CgA is a soluble protein stored and secreted by NETs and has the most clinical utility for diagnosis, prognosis and treatment response. A meta-analysis has shown that CgA has high sensitivity (73%) and specificity (95%) for the diagnosis of NETs. The sensitivity of CgA varies according to the primary site, with higher sensitivity for gastrinomas (100%) and gastric NETs (95%) but lower sensitivity for pancreatic (70%) NETs. CgA is of greater clinical utility in G1 and G2 NETs. An elevated CgA is predictive of shorter survival in small bowel and pNETs. The CgA assay can be falsely elevated by other non-NET factors like proton pump inhibitors (PPI) use and renal impairment.
Serotonin and 5-Hydroxyindoleacetic Acid
Carcinoid syndrome develops from the secretion of 5-hydroxytryptamine (5-HT or serotonin) and other vasoactive peptides. The secreted products from sbNETs can cause a local mesenteric desmoplastic reaction as well as distant fibrosis of the cardiac valves resulting in carcinoid heart disease. 5-HT secretion can be measured directly in serum and platelet assays as well as indirectly from its metabolite 5-hydroxyindoleacetic acid in the urine. Elevated levels correlate with the likelihood of carcinoid heart disease and liver metastases.
Management
Therapies for GEP-NETs are ideally aimed at a cure but also focus on symptom control and antiproliferative effects. The management of NETs requires the use of several different therapies including surgery, biotherapy, chemotherapy, peptide receptor radionuclide therapy (PRRT), and tumor embolization. A multidisciplinary approach is advocated given the multimodal approach to managing NET patients. Patients with advanced disease or recurrence may require several different therapies over their disease therapy, while others may have a more indolent disease and minimal symptoms that are well controlled for years.
Surgery for Primary Neuroendocrine Tumors
Surgery remains the only method for cure and should be considered in all patients if technically feasible. Patients with localized GEP-NET tumors should be offered curative surgical resection of the primary lesion. The surgical management should be individualized for each patient given oncologic and technical considerations as well as comorbidities.
Nonrandomized studies have demonstrated a survival benefit for pNET patients from surgery. Curative surgery in patients with metastatic pNETs offers a survival benefit following resections of primary and metastatic disease. The role of primary resection in the context of unresectable metastatic disease is less clear but there may be some benefit at meta-analysis of nonrandomized studies.
The resection of a primary sbNET and nodal mesenteric mass improves survival. Furthermore, many retrospective studies and meta-analyses have suggested benefit from resecting the primary sbNET in the context of unresectable metastatic disease. Benefit is thought to be due to the reduction in risk of complications, such as small bowel obstruction due to tumor mass effect. Similarly, resection of both primary sbNET and hepatic metastases has an acceptable mortality and morbidity with excellent long-term survival rates.
Liver-directed Therapies
The size, number, and distribution of liver metastases are important factors that affect survival and treatment strategies. Solitary liver metastases can be resected with curative intent and can have 5-year survivals of 100%. Debulking surgery for liver disease should be considered a palliative option in patients with symptoms related to carcinoid syndrome refractory to medical therapy or in whom there is evidence of clinical or radiologic progression of disease. Debulking of greater than 90% of the hepatic tumor burden may lead to longer survival as well as symptom control. However, a Cochrane Review to assess the role of debulking surgery or cytoreductive surgery was equivocal due to the lack of high-quality data.
Locoablative therapy for hepatic metastases
Several interventional radiology and surgical techniques are available for ablation of liver metastases. The methods available include radiofrequency ablation, microwave ablation, cryotherapy, and irreversible electroporation. These methods can be performed percutaneously, laparoscopically, or at open surgery. Radiofrequency ablation series describe a 5-year overall survival of 53% with a local hepatic recurrence rate of 22% and new hepatic lesions in 63% of patients. There are no data to suggest an improvement in overall survival with ablative therapies primarily used for symptom control from reduced hormone secretion.
Hepatic artery embolization
Embolization of the liver can result in necrosis of metastatic tumor tissue resulting in decreased hormonal secretion. Hepatic artery embolization (HAE) can offer good tumor control, although there are limited data on improvements in overall survival. Symptomatic response is seen in 40% to 80% of cases with a biochemical response for hepatic embolization of 7% to 75% and 12% to 75% for hepatic chemoembolization. A study by Gupta and colleagues demonstrated no additional benefit of chemotherapy to transcatheter arterial embolization in metastatic small bowel tumors. Contraindications to performing HAE include portal vein thrombosis, liver failure, and biliary reconstruction as well as a patient’s poor performance status. Postembolization complications include ileus, portal vein thrombosis, hepatic abscess, hepatic fistula, encephalopathy, and renal insufficiency ( Table 4 ).
| Study | Type of NET | HACE or HAE | No. of Patients | Clinical Response (%) | Biochemical Response (%) | Radiological Response (%) |
|---|---|---|---|---|---|---|
| Rusniewski et al, 1993 | Small bowel | HACE | 24 | 73 | 57 | 33 |
| Therasse et al, 1993 | Small bowel | HACE | 23 | 100 | 91 | 35 |
| Clouse et al, 1994 | Small bowel | HACE | 14 | 90 | 69 | 78 |
| Diaco et al, 1995 | Small bowel | HACE | 10 | 100 | — | 60 |
| Roche et al, 2003 | Small bowel | HACE | 14 | 70 | 75 | 86 |
| Kim et al, 1999 | Pancreatic | HACE | 14 | — | 90 | 50 |
| Small bowel | 16 | — | 75 | 25 | ||
| Drougas et al, 1998 | Small bowel | HACE | 14 | 66 | 100 | — |
| Gupta et al, 2005 | Small bowel | HAE or HACE | 69 | — | — | 67 |
| Pancreatic | 54 | 35 | ||||
| Marrache et al, 2007 | Small bowel Pancreatic | HACE | 48 | 91 | 65 | 37 |
| 19 |
Selective internal radiation therapy
Hepatic embolization with Yttrium-90 ( 90 Y) selective internal radiation therapy (SIRT) has been used for over a decade with a small number of studies in NET patients. A study by Kennedy and colleagues in 148 patients with unresectable NET liver metastases demonstrated stable disease in 22.7%, partial response in 60.5%, and complete response in 2.7%. 90 Y microspheres have been better tolerated than chemoembolization in these studies. A recent single-center retrospective series of 40 patients reported an objective tumor response and disease control rates of 54% and 94%, respectively, with a mean overall survival from the first SIRT of 34.8 months.
Medical Therapies for Neuroendocrine Tumors
Several therapy options are available for treating NET patients with advanced or progressive disease with significant survival benefit. An approach is to tailor therapy on the basis of tumor biology, predominantly from primary site, grade, and functional imaging results.
Somatostatin analogues
Somatostatin analogues (SSA) are prescribed on the basis of SSTR expression, most commonly ascertained by tumor avidity from functional imaging assessment. Octreotide was the first synthetic analogue of somatostatin developed and reduces hormonal secretion. The original preparation has a short half-life requiring intravenous or 3-times-daily subcutaneous dosing regimen to maintain steady levels. Long-acting preparations (28 days) have been developed, but short-acting analogues are useful for breakthrough symptoms and in the perioperative period.
Moreover, long-acting analogues (LAR) have been demonstrated to have an antiproliferative effect. A randomized placebo-controlled prospective study in patients with metastatic sbNETs demonstrated that long-acting octreotide inhibited tumor growth and delayed time to progression. A further study in GEP-NETs (CLARINET) using lanreotide confirmed the antiproliferative effects of SSAs with significantly prolonged progression-free survival (PFS; median not reached vs median of 18.0 months, P <.001).
Tolerance to SSAs is a recognized phenomenon, and there is a need for new biotherapy agents. Pasireotide, a new multiligand SSA, has completed a phase III study to assess control of functional symptoms in patients with metastatic NET. In this study, pasireotide LAR was compared with octreotide LAR in patients with uncontrolled NET symptoms and did not demonstrate any significant improvement in symptoms. However, a median investigator-assessed PFS of 11.8 months for pasireotide (vs 6.8 months for octreotide) was observed (hazard ratio = 0.46; P = .045).
Interferon-α
Interferon therapy has been used since 1982 for symptom control with 50% to 60% of carcinoid syndrome patients experiencing a reduction in flushing and diarrhea. Significant biochemical responses are reported in 40% to 50% of cases. Its mechanism of action is unclear, although it is thought to act through antisecretory and immunomodulatory functions.
Chemotherapy
Chemotherapy has been widely used as first-line therapy for unresectable poorly differentiated NETs and well-differentiated pNETs. The results from different pNET chemotherapy regimens are variable. The response rates demonstrated by Moertel nearly 40 years ago have been difficult to replicate in recent studies. Most chemotherapy trials have been single-center retrospective series. However, the multicenter randomized prospective study NET-01 study reported recently on the addition of cisplatin to capecitabine and streptozocin (STZ) in GEP-NETs patients. It demonstrated no benefit of adding cisplatin to a capecitabine and STZ regimen. The disease control rate was 80% with the medical PFS of 10.2 months. STZ-based combinations are an accepted first-line chemotherapy regimen for well-differentiated G1 or G2 pNETs.
In well-differentiated pNETs, there is also evidence emerging regarding the use of Temozolomide, either as monotherapy or in combination with capecitabine. A retrospective study of temozolomide combined with capecitabine in 30 chemotherapy-naive patients demonstrated an objective radiographic response rate of 70% and a median PFS of 18 months. In contrast, the overall response rate for sbNETs to a range of chemotherapy agents is less than 30% ( Table 5 ).






