Fig. 6.1
Magnetic resonance angiogram showing anatomy of the splenic and renal arteries. (Reproduced with permission from Humar A, Khwaja KO, Sutherland DER. Pancreas transplantation. In: Humar A, Matus AJ, Payne WD, eds. Atlas of organ transplantation. New York; Springer; 2006)
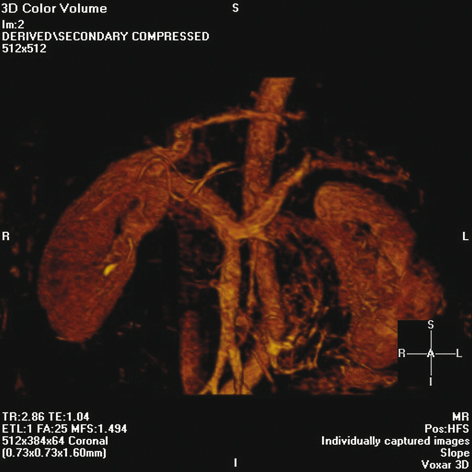
Fig. 6.2
Magnetic resonance angiogram showing anatomy of splenic vein and portal confluence. (Reproduced with permission from Humar A, Khwaja KO, Sutherland DER. Pancreas transplantation. In: Humar A, Matus AJ, Payne WD, eds. Atlas of organ transplantation. New York; Springer; 2006)
Operative Considerations
The Donor Operation
Detailed discussion of the operative technique is beyond the scope of this discussion. Procurement of the pancreas (and kidney, in the case of combined kidney–pancreas transplant) may be performed using open or laparoscopic techniques. The decision to perform one or the other is dependent on the surgical experience of the center. Generally, the laparoscopic approach is preferred due to the superior cosmetic outcome and more rapid convalescence of the donor.
In general, we try to preserve the spleen in order to prevent the potential immunologic sequelae associated with a splenectomy, such as overwhelming postsplenectomy sepsis (OPSS) . In a series of five laparoscopic donors at the University of Minnesota [14], splenectomy was performed because of a nonviable spleen that was recognized at the time of surgery. Based on the open donor pancreatectomy data, there is an 8.5–25 % rate of splenectomy [15, 16]. At the current stage of evolution of this technique, we prefer the hand-assisted approach, because having tactile feedback greatly facilitates safe dissection and partially overcomes the lack of three-dimensional visualization inherent in laparoscopy.
Postoperative Care of the Donor
Postoperative care of the donor is similar to that of any patient undergoing major abdominal surgery. A nasogastric tube is left in place until return of bowel function. Hemoglobin, serum amylase, lipase, and glucose levels are followed serially. Persistently elevated amylase and lipase levels suggest pancreatitis, a leak, or pseudocyst formation. Persistent or severe left upper quadrant or left shoulder pain should be evaluated with CT and 99mTc-sulfur-colloid scan of the spleen to assess splenic viability. If the spleen appears infarcted, a splenectomy should be performed.
Donor Outcomes
Open Donor Pancreatectomy
A retrospective study from the University of Minnesota from January 1978 to August 2000 reviewed 115 open LD pancreas transplants : 51 pancreas transplant alone (PTA), 32 pancreas after kidney (PAK), and 32 SPK [16]. There were no donor mortalities. Donor complications can include hemorrhage, splenic infarct, abscess, pancreatitis, and pseudocyst formation. Splenectomy was required in 8 % of donors. A recent review documents a rate of splenectomy of 5–15 % [17]. Pseudocysts occurred in 10 % and percutaneous drainage was necessary in 60 %. Within the subset of SPK donors , 20 % required perioperative blood transfusions. Two donors required percutaneous drainage of a noninfected peripancreatic fluid collection [18]. The median donor age was 44 years (range 26–49). The median operative time was 6.9 hours with a median length of stay of 8 days (range 6–24) [16].
Long-term follow-up was possible in 67 patients. The remaining 48 could not be located, refused to participate, lived outside of the US, or were deceased. Ten donors had abnormal HbA1c levels. Three of them required insulin > 6 years postoperatively. One of these donors had a history of gestational diabetes. The other two had predonation BMI > 27 kg/m2. Consequently, gestational diabetes and elevated BMI are now considered contraindications to donation. Hyperglycemia, however, may have occurred secondary to development of type 2 diabetes. Since 1996, all donors have maintained normal HbA1c levels (4.9 –6.2 %) following these guidelines [16].
Laparoscopic Donor Pancreatectomy
From March 1999 to August 2003, five laparoscopic pancreatectomies were performed at the University of Minnesota [14]. The mean donor age was 48.4 years ± 8.7 with a BMI of 23.7 kg/m2 ± 3.0. The mean length of surgery for PTA donors was 4.5 ± 0.13 hours and for SPK donors , 7.9 ± 0.38 hours. Mean blood loss was 330 mL ± 228. Once the learning curve has been overcome, however, the laparoscopic approach may actually have shorter operative times, as less dissection is required compared to the open technique. In two of the SPK cases, the donor surgical team had to wait 1.5–2 hours for the recipient team to receive the organs, thus prolonging the operative time for the donor. One splenectomy had to be performed at the time of the donor surgery for a nonviable spleen. No pancreatic leaks or pancreatitis was observed. The average serum glucose was 112 mg/dL ± 11.7 upon discharge. The amylase and lipase on discharge were 72.2 U/L ± 26.3 and 67.2 U/L ± 34.0, respectively. None of the donors have required oral antidiabetic medications or insulin. At 3 years follow-up, the mean postoperative HbA1c was 5.7 % ± 0.2. One donor refused biochemical follow-up. Postoperative stay for the laparoscopic donors was 8 days ± 2. No obvious statistical advantage was observed in terms of decreased hospital stay. However, based on the first handful of laparoscopic cases performed, this may be a function of over-vigilance on the part of the treating team as tends to occur with new procedures. Certainly, in laparoscopic nephrectomy, the advantages of reduced hospital stay and earlier postoperative recovery have been demonstrated [19, 20]. All the donors have reported that they are back to their preoperative state of health and working. Satisfaction was high in terms of cosmetic result because the donor operation can be performed through a relatively small midline incision and only two trocar sites (Fig. 6.3).
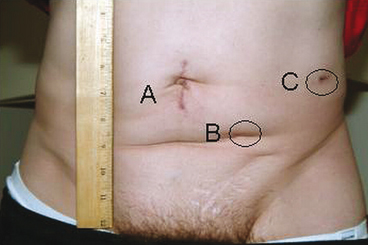
Fig. 6.3
Surgical incisions 3 weeks post laparoscopic distal pancreatectomy and left nephrectomy. a Gelport site; b 12-mm camera port site; c 12-mm instrument port site. (Reproduced with permission from Humar A, Khwaja KO, Sutherland DER. Pancreas transplantation. In: Humar A, Matus AJ, Payne WD, eds. Atlas of organ transplantation. New York; Springer; 2006)
Recipient Outcomes
LD pancreas recipient survival rates were 93 and 90 % at 1 and 5 years, respectively. In the pretacrolimus epoch, technically successful PTA and PAK recipients had a graft survival rate of 68 and 50 %, respectively. The immunologic advantage of LD pancreas transplants , at that time, was clear. Only 13 % of LD pancreas recipients had graft loss secondary to rejection, whereas 41 % of cadaver organ recipients lost their grafts from rejection [16]. In the current immunosuppressive era, this difference hardly exists owing to modern drug regimens (tacrolimus, mycophenolate mofetil), improved operative techniques, and aggressive postoperative anticoagulation. Consequently, although solitary LD pancreas transplants are still performed, the focus is primarily on SPK donors in order to address the shortage of cadaver organs in this subset of pancreas transplant recipients.
Thirty-two open LD SPK transplants were performed at the University of Minnesota from 1994 to 2000 [16]. Patient and kidney graft survival was 100 % at 1 year. Pancreas allograft survival was 87 %. This compares favorably to current cadaver SPK transplant data that demonstrates 84 % 1-year survival for pancreas grafts and 90 % kidney allograft survival [21]. This marked improvement in patient and graft survival, compared to our series of LD PTA and PAK transplants before 1994, may be attributed to improved immunosuppression, routine postoperative anticoagulation (perioperative low-dose heparin and long-term aspirin), and better infection prophylaxis (e.g., gancyclovir) [16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






