Fig. 10.1
Setting-up staging laparoscopy. The laparoscopic monitor is placed beyond the patient in the direction the surgeon is working (a). The laparoscopic ultrasound machine is placed to the left of this. An assistant is controlling the laparoscopic camera while the primary surgeon manipulates the ultrasound probe. Note the remote control unit for the ultrasound machine in the surgeon’s left hand with which he is recording images and turning the Doppler flow on and off. There are many options for port placement and this is our preferred (b). A pneumoperitoneum is established through an infraumblical port inserted with an open technique. A further 10–12 mm port is placed in the epigastrium to the left of the midline and well below the costal margin. A 5 mm port for a grasper is placed in the right upper quadrant
There are a number of options for laparoscopic port placement, and this depends on the primary organ for investigation. Our standard approach (Fig. 10.1b, c) involves establishing a pneumoperitoneum (12 mmHg) via a 10 mm infraumbilical port placed under direct vision. A further 10 mm port is placed in the epigastrium to the left of the midline well below the costal margin. A 5 mm port is usually placed on the right side for use of a grasper. These positions allow easy access to the liver, gallbladder and portal pedicle. In the staging of oesophagogastric cancer, it can be preferable to place the 10–12 mm port on the right side of the abdomen and the 5 mm port on the left. The right-sided and umbilical ports can then be used to gain easy access to the stomach and oesophageal hiatus.
A 30° laparoscope is inserted through the umbilical port and a careful inspection of the intra-abdominal organs and peritoneum performed (Fig. 10.2a, b). Particular attention is paid to the falciform ligament, liver (including the under surface, Fig. 10.2a), diaphragm, hepatoduodenal ligament and lesser omentum. The greater omentum is retracted superiorly to allow the small bowel mesentery and ligament of Treitz to be directly visualised.
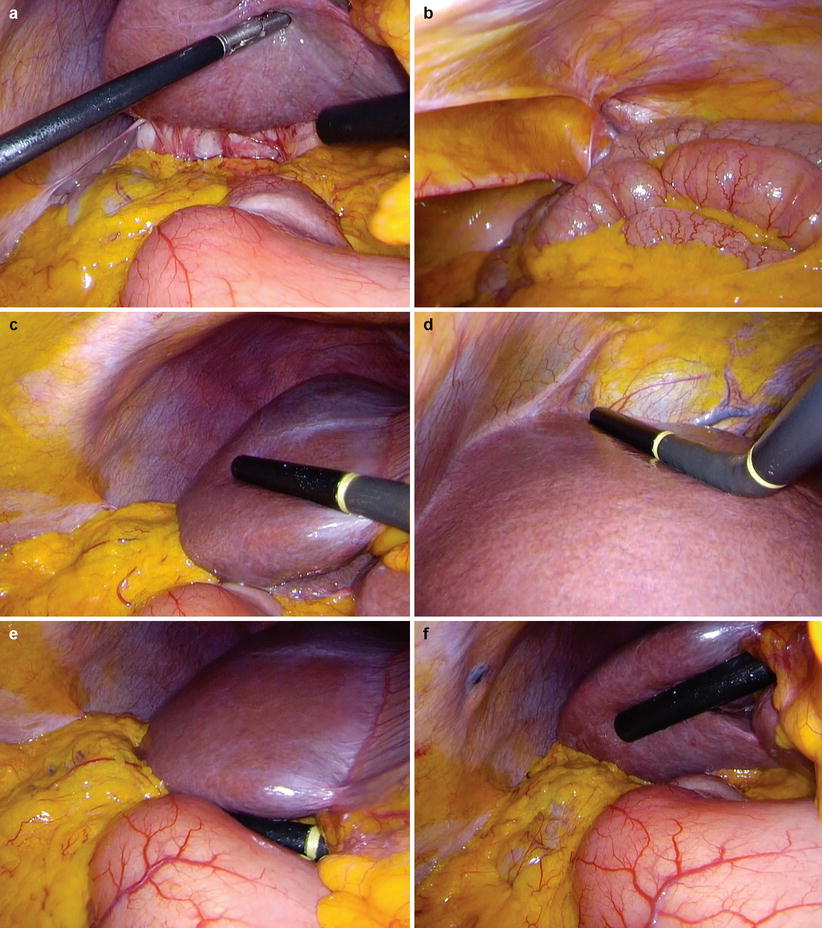

Fig. 10.2
Staging laparoscopy. Begin with a full inspection of the peritoneal cavity lifting the left and right liver to view the underside (a) and paying particular attention to the peritoneum (b). Use laparoscopic ultrasound to orientate on the origin of the left and right portal vein branches and carefully visualise the parenchyma of the right lobe (c). Look on the left side of the falciform ligament and scan segments II and III (d). Place the probe on the hepatoduodenal ligament in the transverse plane to examine the portal pedicle (e). The probe can also be placed on the underside of any part of the liver and orientated anteriorly (f). Adhesions around the gallbladder can be seen as a result of recent acute cholecystitis (a)
Laparoscopic Ultrasonography
A high-resolution flexible tip linear array transducer is inserted through the epigastric port (Fig. 10.2c). Systematic scanning of the liver should start with identification of standard landmarks and of the liver parenchyma. A window may be made in the falciform ligament to aid visualisation of the hepatic outflow. Intrahepatic liver metastasis can appear as hyper-, iso- or hypoechoic lesions on imaging. It can also be useful to position the probe on the underside of the liver, particularly on the right lobe (Fig. 10.2f). This manoeuvre can be used to better visualise lesions in the posterior section (segments VI/VII) of the liver.
Depending on the location of the primary tumour, identification of structures in the portal triad is now performed (Fig. 10.2e). Visualisation of the portal structures can be aided by inserting the probe through the infraumbilical port and placing it on the hepatoduodenal ligament (see Chap. 14 for further information). By identifying the inferior vena cava posteriorly and rotating the probe counterclockwise, the portal vein, bile duct and hepatic artery are visualised. The portal vein can be followed to the splenoportal confluence and continued down the superior mesenteric vein. This manoeuvre is clearly important in tumours of the head of pancreas and distal common bile duct.
Vascular invasion is suggested by the absence of the tissue plane between the tumour and blood vessel. Avoid excessive pressure with the probe as this can emulate the appearance of tumour involvement into vessels when this has not occurred. The presence of a fixed stenosis in a vessel in more than one plane suggests tumour involvement. If views are not adequate due to poor probe contact or as a result of the pneumoperitoneum, CO2 can be released and saline injected into the peritoneum to improve probe contact, though is rarely required.
In pancreas and biliary tumours, the primary lesion should be assessed to determine the proximal and distal extent, radial extension (particularly arterial and venous invasion) and the presence of lymph node metastases. Lymph nodes invaded by tumour are hypoechoic with a loss of definition. While enlarged lymph nodes may represent the presence of metastatic disease, this finding is non-specific and should be confirmed pathologically (Fig. 14.4b, c).
Careful examination should be made of the coeliac trunk and aortocaval window for suspicious nodes. Large coeliac trunk nodes can sometimes be accessed through the less omentum and biopsy made. Care needs to be taken to avoid bleeding in this area. Colour Doppler can be helpful in differentiating vessels from nodes during these manoeuvres (Fig. 10.3).
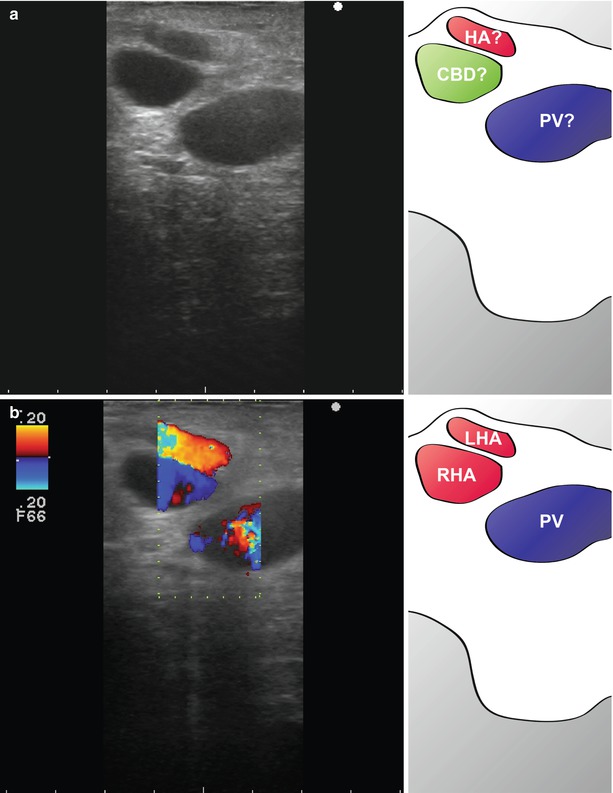

Fig. 10.3
Colour flow Doppler in the identification of structures. Three structures are apparent in the hepatoduodenal ligament (a). These appear to have the configuration of the portal vein (?PV), hepatic artery (?HA) and bile duct (?CBD) (see Chap. 14). However, when colour flow Doppler is used, it is apparent that all three structures (LHA left hepatic artery, RHA right hepatic artery, PV portal vein) are blood vessels (b). In this patient, the hepatic artery bifurcates early, and the bile duct was small and lateral. Colour flow was useful in differentiating the bile duct from the artery
Laparoscopic Biopsy
A patient presenting with painless obstructive jaundice and a mass in the head of pancreas on CT underwent staging laparoscopy (Fig. 10.4). A full staging laparoscopy was performed followed by laparoscopic ultrasound through an epigastric port (Fig. 10.4a, b). Suspicious lesions were seen in segment II/III (Fig. 10.4a, c) and segment V (Fig. 10.4b, d) of the liver. These were firm on direct pressure with a grasper and had a ‘target’ appearance on ultrasonography suggesting metastatic disease.
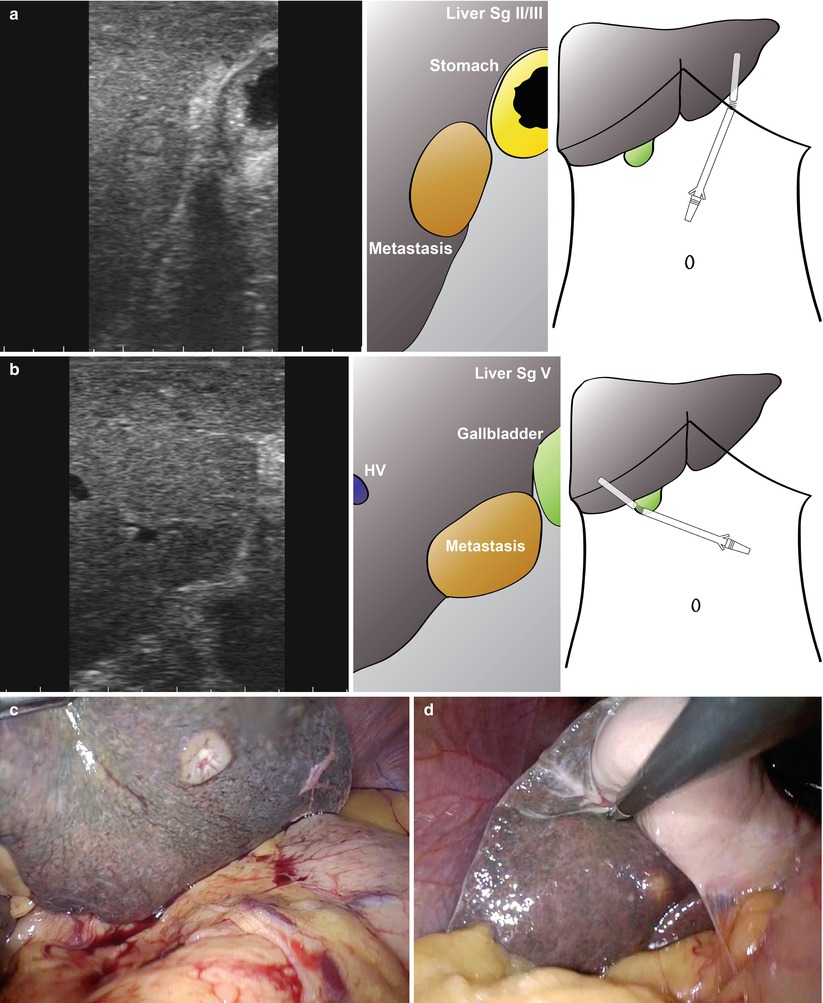

Fig. 10.4
Staging laparoscopy in a patient with a head of pancreas mass. A small lesion had been seen in the right liver on CT and MRI but was too small to characterise. At laparoscopy a lesion was seen in segment II/III of the liver (a, c) and segment V (b, d). These looked suspicious and on biopsy with frozen-section analysis, the left-sided lesion was confirmed to be adenocarcinoma (c)
Any suspicious lesions identified can then be biopsied directly or using ultrasound guidance. The lesion in segment II/III was accessible and biopsied directly using scissors (Fig. 10.5). It is best not to use diathermy when taking the biopsy to avoid thermal artefact, particularly when the specimen is small. Bleeding can be controlled with diathermy after the specimen is removed (Fig. 10.5f). In this case, the biopsy was sent for direct frozen-section analysis and adenocarcinoma was confirmed.
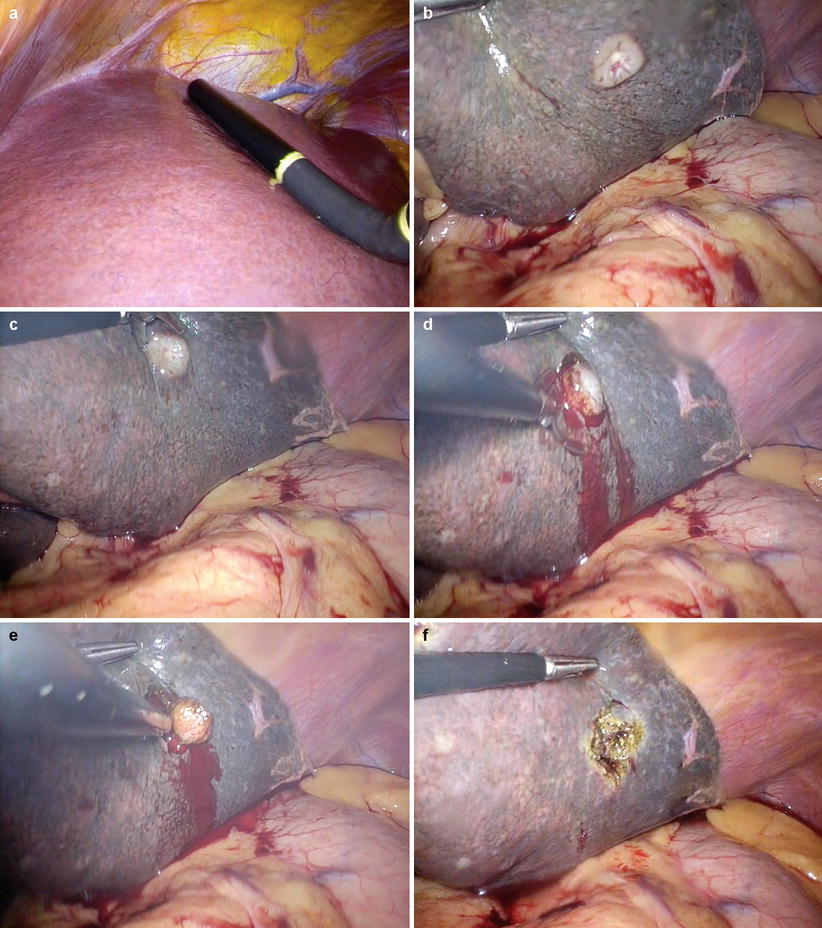

Fig. 10.5
Laparoscopic ultrasound and biopsy of suspicious liver lesion in a patient with head of pancreas mass. The lesion is identified with the ultrasound (a) and visualised (b). Using scissors without diathermy (c), the lesion is excised and subjected to frozen-section analysis (d, e). Diathermy is used to achieve haemostasis (f)
Oesophagogastric Junctional Cancer and Gastric Cancer
The epidemiology of gastric cancer has altered over the last 40 years. The site of origin within the stomach has changed in frequency in the USA and Europe, with a reduction in the incidence of cancer arising in the distal half of the stomach and a rapid increase in the number of cases of the cardia and gastro-oesophageal junction [1]. The overall incidence of cancer at these sites has also risen rapidly, especially in patients younger than 40 years.
The prognosis of patients with these cancers is related to local tumour extent, including both nodal involvement and direct tumour extension beyond the gastric wall [2]. Importantly, the presence of metastases in the peritoneal cavity or distant organs renders the disease incurable and the prognosis is usually very poor. Following resection, the peritoneum is the most common site of recurrence as a result of malignant cells shed from the primary tumour [3].
Radical surgical resection is the only curative treatment for patients with oesophagogastric cancers and is the first-choice treatment in patients with early-stage disease [4]. However, the majority of patients have advanced disease at the time of diagnosis, and accurate preoperative staging is essential to guide management. The objectives of cancer staging are to confirm the diagnosis of malignancy and to determine the extent of the disease, enabling the most appropriate treatment modality to be selected [5]. Given that radical surgery provides the only curative treatment of oesophagogastric cancer, the number of falsely over-staged patients must be minimised to ensure that the possibility for cure is not missed. Yet the sensitivity of any test measuring dissemination of oesophagogastric cancer must be high to avoid unnecessary explorative laparotomies. It has been widely shown that despite improvements in quality, CT still has a low sensitivity for detecting peritoneal disease, hence the proposed need for laparoscopic staging [6].
High-quality contrast-enhanced computerised tomography is the most accurate, widely used, non-invasive modality for detecting distant metastases in oesophagogastric cancer [7]. The introduction of endoscopic ultrasound (EUS) has also been successful in improving the accuracy of preoperative staging in oesophagogastric cancers and is more accurate than CT in determining T and N stages [8] (see Chap. 11 for further information). An added benefit of EUS is the ability to sample suspicious lymph nodes using needle aspiration/mini-core biopsy. As in other diseases described here, CT is inaccurate in the evaluation of peritoneal disease, with sensitivity ranging from 30 to 73 % and specificity from 83 to 100 % [9].
A number of small observational studies have been published comparing imaging modalities in the staging of oesophagogastric cancer. In a study from the authors’ own centre, LUS was compared with CT and EUS. Thirty-six patients with histologically proven carcinoma of the oesophagus or stomach who were considered fit for surgery underwent CT, EUS and LUS. The findings of these investigations were compared with final histopathology or intraoperative findings where the tumour was irresectable. Locally advanced tumours were accurately identified by CT in 15/16 (94 %), EUS in 14/16 (88 %) and LUS in 10/12 (83 %). In the assessment of locoregional lymph node involvement, EUS was superior to both CT and LUS: accuracy 21/29 (72 %) versus 17/29 (59 %) and 17/29 (59 %). Although the specificity of LUS in assessment of lymph node involvement was good compared to CT and EUS, the sensitivity was poor: sensitivity/specificity, EUS 79 %/60 %, CT 68 %/40 % and LUS 42 %/90 %. LUS was clearly superior in the identification of metastatic disease, with an accuracy of 21/32 (81 %) versus 23/32 (72 %) for CT. The authors concluded that although the numbers were small, CT, EUS and LUS act in a complimentary manner to provide the most complete preoperative staging for patients with oesophagogastric cancer [10].
In an earlier study aiming to determine the added benefit of LUS over laparoscopy, of 93 patients who underwent laparoscopy, 18/93 (19.4 %) were shown to have irresectable disease and avoided an inappropriate laparotomy. With the addition of LUS, a further 7/93 (7.5 %) were found to have advanced disease [11]. The unnecessary laparotomy rate reduced from 5/25 (20 %) in those without laparoscopy to 9/75 (12 %) with laparoscopy and 2/68 (3 %) in those who had LUS. These findings suggesting a positive role for LUS in the staging of OG malignancy were mirrored in other studies [12–15]. However, more recent studies have shown less benefit with the addition of LUS to laparoscopy, possibly as a result of the improved quality of CT and EUS.
In one such study comparing CT, transabdominal ultrasound, laparoscopy and LUS in the assessment of 47 patients with gastric cancer, laparoscopy was accurate in determining overall clinical stage (31/37, 84 %) compared with transabdominal ultrasound (20/37, 54 %) and CT (23/37, 62 %) [6]. However, the addition of laparoscopic ultrasonography did not change the stage of the disease or the decision of whether to proceed with laparotomy for any of the patients, which was correctly predicted in 95 % of the cases. Laparoscopy was superior for detecting peritoneal seeding and ascites, characteristic features of advanced gastric carcinoma. Laparoscopy was also superior in identifying local extension of gastric carcinoma, but the additional information from LUS was minor. The lack of benefit with LUS was also seen in another smaller study, which looked at 18 patients [16].
One of the advantages of staging laparoscopy is the ability to sample tissues. Specifically in gastric cancer, the use of peritoneal lavage cytology can be used to determine operability. In this procedure, fluid is instilled in the upper abdominal cavity at laparoscopy, aspirated and spun-down for cytological examination. In a study examining the value of this procedure in oesophagogastric adenocarcinoma, 255 patients had peritoneal washings at laparoscopy of which 48/255 (18.8 %) had overt peritoneal metastases at staging laparoscopy. Of the remaining patients, 15/207 (7.2 %) had positive cytology. These patients had a median (95 % confidence interval) survival of 13 (3.1–22.9) months versus 9 (7.4–10.6) months for those with overt peritoneal metastases (p = 0.517). The authors conclude that positive peritoneal cytology in the absence of overt peritoneal metastases was a marker of poor prognosis and should be considered to signify incurable disease [17].
Overall, laparoscopy increases the demonstrated disease stage in 40 % of gastric cancer patients and avoids unnecessary laparotomy in around 25 % (Level II evidence) [18]. It has also been suggested that laparoscopy may downstage tumours thought to be T4 on EUS to T3 by demonstrating the absence of direct invasion into surrounding structures [18]. Early series showed the addition of LUS to be an advantage, but this has been questioned in more recent studies. While the requirement for staging laparoscopy with peritoneal washings is supported by the NCCN Clinical Practice Guidelines in the USA [19] and the SIGN guidelines in the UK [7], neither specifically recommend the use of LUS. The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines conclude that LUS for gastric cancer staging can be performed safely and adds little time to the duration the procedure (Grade A). The routine use of staging laparoscopy and LUS after a negative preoperative workup is recommended (Grade B) [18].
Colorectal Liver Metastases
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most frequent cause of cancer death in the USA [20]. The liver is the most frequent site of metastases, and liver resection and ablation are accepted as standard treatment strategies [21]. Outcomes after surgical management of colorectal liver metastases (CRLM) are improving, with 5-year survival now approaching 50 % [22]. Accurate preoperative staging is essential if treatments are to be targeted to those who will benefit most.
Factors associated with poor long-term outcome after liver resection for CRLM include positive margin (hazard ratio (HR) = 1.7, p = 0.004), extrahepatic disease (HR = 1.7, p = 0.003), node-positive primary (HR = 1.3, p = 0.02), disease-free interval from primary to metastases < 12 months (HR = 1.3, p = 0.03), number of hepatic tumours > 1 (HR = 1.5, p = 0.0004), largest hepatic tumour > 5 cm (HR = 1.4, p = 0.01) and carcinoembryonic antigen level > 200 ng/ml (HR = 1.5, p = 0.01) [23].
Laparoscopy or LUS is not generally recommended for the staging of patients with CRC without evidence of CRLM. In patients with CRC and suspected or proven CRLM, US and European guidelines recommend staging with CT with intravenous contrast, positron emission tomography (PET) CT and/or MRI imaging [21, 24]. As the quality and resolution of these modalities improve, the role of laparoscopy and laparoscopic ultrasound in the staging of colorectal cancer becomes less clear.
The UK guidelines suggest that patients with ‘high-risk’ primary disease (T4 (perforated), C2 (apical node)) should have careful preoperative investigations that might include laparoscopy. Laparoscopy may identify occult metastatic disease and prevent unnecessary laparotomy in some patients with potentially resectable colorectal liver metastases, and LUS may provide additional information in selected patients [21]. Laparoscopy/LUS may also be useful in the presence of multiple bilobar disease when there are concerns regarding the feasibility of liver resection or imaging is indeterminate.
Conversely, Dutch guidelines for the management of CRLM state that there is no role for diagnostic laparoscopy in routine daily practice due to its invasiveness and the low prevalence of small subcapsular liver lesions and extrahepatic disease. Small liver metastases ‘missed’ on preoperative imaging also have less clinical consequence as these can generally be resected [25].
A recent meta-analysis examined the role of laparoscopy and laparoscopic ultrasound in the preoperative staging of patients with resectable colorectal liver metastases (Tables 10.1 and 10.2) [38]. The authors identified 12 studies that described a total of 1,047 patients who underwent staging laparoscopy and/or LUS. The difficulty in comparing studies of this type is the assessment of inclusion criteria for the diagnostic test. Significant heterogeneity exists between studies making data synthesis difficult. Clearly, older studies using low-resolution CT imaging are likely to have less contemporary relevance, while trials using the best available cross-sectional imaging with tighter inclusion definitions are likely to demonstrate the greatest utility in laparoscopy, at the expense of the total proportions of their practice numbers included. None of the considered trials were randomised or blinded. The true yield of laparoscopy/LUS for CRLM was 19 % (95 % CI, 16–22 %) with an overall sensitivity of 59 % (95 % CI, 53–65 %). Subgroup analysis for detection of other liver and peritoneal lesions showed a sensitivity of 59 % (95 % CI, 49–67 %) and 75 % (95 % CI, 63–85 %), respectively.
Table 10.1
Meta-analysis of studies examining the role of laparoscopy and laparoscopic ultrasound in the preoperative assessment of patients with resectable colorectal liver metastases: study characteristics
Study | Year | Country | Study type | Preoperative investigation | Patients, n | Median age, years | STARD scorea |
|---|---|---|---|---|---|---|---|
Biondi [26] | 2010 | Italy | Not defined | CT/MRI/PET | 65 | 63 | 10 |
Muntean [27] | 2009 | Romania | Prospective | CT/MRI/PET | 18 | NA | 13 |
Pilkington [28] | 2007 | England | Retrospective | CT/MRI | 73 | NA | 12 |
Khan [29] | 2007 | England | Retrospective | CT/MRI | 210 | NA | 8 |
Mann [30] | 2007 | England | Retrospective | CT/CEA/PET/MR | 200 | 60 | 13 |
Mortensen [31] | 2006 | Denmark | Retrospective | CT | 45 | 62 | 13 |
de Castro [32] | 2004 | The Netherlands | Prospective | US/CT/MR | 43 | NA | 15 |
Koea [33] | 2004 | New Zealand | Prospective | CT/CEA | 59 | 65 | 14 |
Metcalfe [34] | 2003 | Australia | Retrospective | CT/CEA | 24 | NA | 12 |
Grobmyer [35] | 2004 | United States | Retrospective | CT/CEA/PET/MR | 264 | 62 | 16 |
Gholghesaei [36] | 2003 | The Netherlands | Retrospective | CT/CEA/PET/MR | 56 | NA | 16 |
Rahusen [37] | 1999 | The Netherlands | Not defined | CT/CEA/US/MR | 50 | 61 | 14 |
Overall | – | – | – | – | 1,107 | – |
Table 10.2
Meta-analysis of studies examining the role of laparoscopy and laparoscopic ultrasound in the preoperative assessment of patients with resectable colorectal liver metastases: study results
Study | Lap | Lap/LUS | Sensitivity, % | Specificity, % | PPV, % | NPV, % | True yield, % |
|---|---|---|---|---|---|---|---|
Biondi [26] | 62 | 62 | 72.7 (54.50–86.70) | 100 | 100 | 76.3 | 38.7 |
Muntean [27] | 18 | 18 | 75.0 (19.4–99.40) | 92.9 (66.10–99.80) | 75 | 92.8 | 16.6 |
Pilkington [28] | 73 | 73 | 69.6 (47.10–86.80) | 100 | 100 | 87.7 | 21.9 |
Khan [29] | 202 | 202 | 39.3 (21.50–59.40) | 100 | 100 | 91.1 | 5.45 |
Mann [30] | 178 | 178 | 61.9 (48.80–73.90) | 100 | 100 | 82.7 | 21.9 |
Mortensen [31] | 38 | 38 | 57.1 (18.40–90.10) | 100 | 100 | 91.1 | 10.5 |
de Castro [32] | 32 | 32 | 71.4 (29.00–96.30) | 100 | 100 | 92.5 | 15.6 |
Koea [33] | 54 | 41 | 37.5 (8.50–75.00) | 100 | 100 | 90.2 | 7.3 |
Metcalfe [34] | 24 | 24 | 66.7 (34.90–90.01) | 100 | 100 | 75 | 33.3 |
Grobmyer [35] | 264 | 168 | 41.3 (29.0–54.0) | 100 | 100 | 84.4 | 15.4 |
Gholghesaei [36] | 55 | 48 | 73.1 (52.50–88.40) | 100 | 100 | 80.5 | 39.5 |
Rahusen [37] | 47 | 47 | 75.0 (53.30–90.20) | 100 | 100 | 79.3 | 38.3 |
Overall | 1,047 | 931 | 59.1 (53.20–64.70) | 99.9 (99.30–100) | 99.4 | 86 | 18.90 (16.44–21.57) |
The use of a clinical risk score (CRS) may be a valid method of identifying patients most likely to benefit from LUS. In a study of 79 patients, LUS prevented unnecessary laparotomy in 15/74 patients by predicting the benign nature of lesions or demonstrating unresectability [39]. A CRS was determined based on lymph node-status of primary tumour, disease-free interval, number of metastases, largest metastasis and CEA. In those with a CRS < 2, LUS prevented a laparotomy in only 7 % of patients. However, in those with a CRS > 2, LUS prevented an operation in 24 % of patients. The authors concluded that selecting those likely to benefit from LUS will increase the utility of the investigation.
The best indication for laparoscopy and laparoscopic ultrasound in CRLM is in patients with resectable disease, but a suspicion of peritoneal disease that is not well defined on cross-sectional imaging or PET-CT. This is with the intention of avoiding an unnecessary laparotomy in patients with extrahepatic disease. As laparoscopic liver resection becomes more common, this step will be part of the resection procedure anyway.
The final area in which LUS may be of use is in patients with borderline resectable disease. Figure 10.6 shows images from a patient with a colorectal liver metastasis high in segment VIII. A right hepatectomy was being considered, but significant progression of disease had occurred since the last cross-sectional imaging. LUS shows clear impingement of the metastasis upon the middle hepatic vein. The procedure was abandoned, and the patient had radiological embolisation to the right lobe and segment IV. A successful extended right hepatectomy was performed 6 weeks later. In a different case, local resection was being considered for a right-sided CRLM (Fig. 10.7). Again, there has been significant progression of disease and with ultrasonography clearly showing tumour abutting the segment VIII pedicle (Fig. 10.7a, b, Video 10.1) and indenting the right hepatic vein with possible invasion (Fig. 10.7c, d, Video 10.2). This patient went on to have a successful open right hepatectomy.
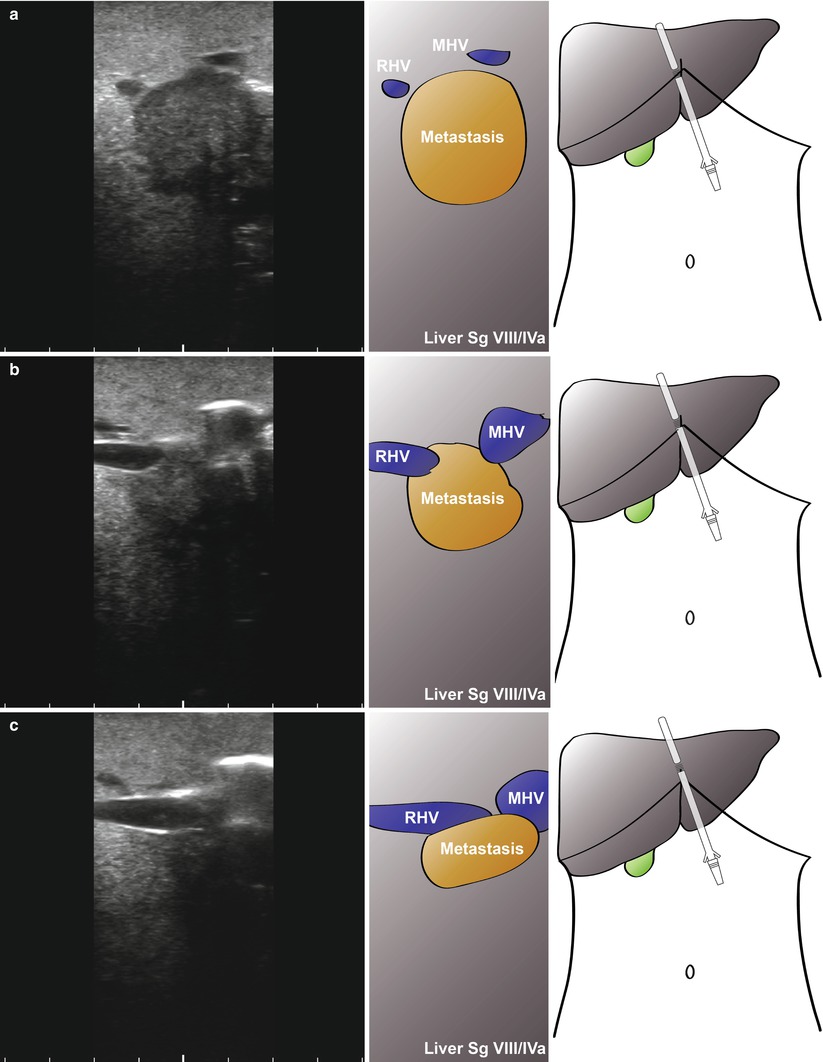
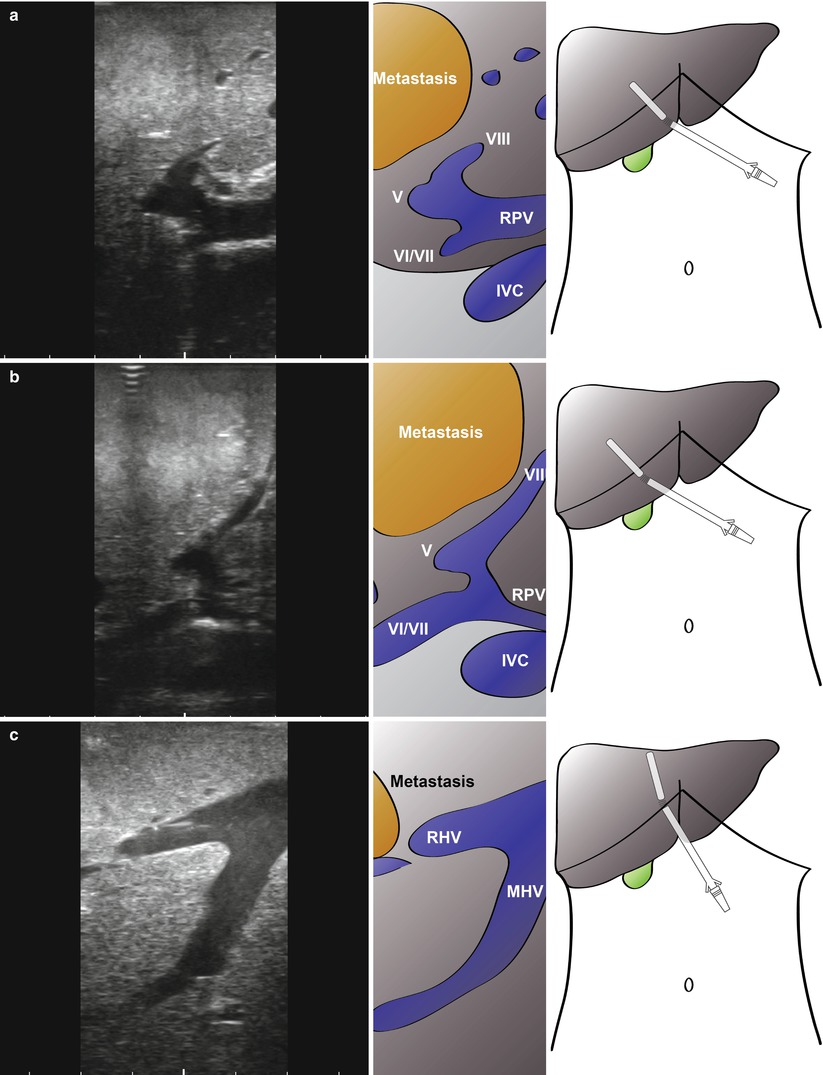
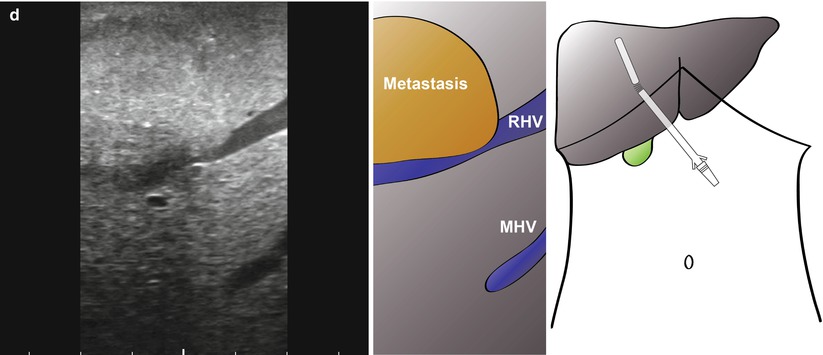

Fig. 10.6
Laparoscopic ultrasonography in a patient with large colorectal liver metastasis at the hepatic outflow (a; RHV, right hepatic vein; MHV, middle hepatic vein). As the probe is moved up towards the suprahepatic vena cava (b), a large metastasis can be seen sitting between the right and middle hepatic veins (c)


Fig. 10.7
Intraoperative ultrasonography to determine relationship of large colorectal liver metastasis to vascular structures. A local resection was being considered, but ultrasonography clearly shows the tumour abutting the segment VIII pedicle (a, b, Video 10.1) with no resection margin possible at this structure. In addition, there is indenting and possible invasion of the right hepatic vein (c, d, Video 10.2; RHV). The patient has a successful open right hepatectomy. MHV, middle hepatic vein; IVC, inferior vena cava; V, segment V pedicle; VI/VII, segement VI/VII pedicle
In summary, the ratio of patients benefiting from laparoscopy/LUS (i.e. those avoiding an unnecessary laparotomy) to those submitted to the procedure (the true yield) is around 20 %. It is likely that this could be increased by selecting the patients most likely to benefit. Ultrasound has a place in the assessment of borderline resectable disease, and the rise in the number of laparoscopic liver resections will naturally encourage an increased use in LUS assessment.
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide [40]. The incidence is particularly high in East Asia and sub-Saharan Africa and is rising in North America and Europe. Around 80 % of cases develop on a background of chronic liver disease with aetiology varying by geography and the primary factor being infection with hepatitis B or hepatitis C virus [41]. Liver resection and liver transplantation provide an opportunity for cure. Ablation of lesions and transarterial chemoembolisation can be used to control disease while awaiting transplantation or as palliative measures in advanced disease. The role of chemotherapy has expanded in recent years and will likely become more prominent in the future.
Staging of HCC is important in determining suitability for transplantation. The original ‘Milan criteria’ of one lesion smaller than 5 cm or 3 lesions smaller than 3 cm, together with no extrahepatic disease or vascular invasion, have been broadened [42]. Patients with no cirrhosis or good preservation of liver function are considered for resection. The use of magnetic resonance imaging (MRI) with liver-specific contrast agents has become the primary mode of investigation of suspected HCC [43]. The requirement, therefore, of laparoscopic staging of HCC is now limited. In centres without access to MRI, LUS can still be useful in guiding treatment decisions [41]. Figure 14.8 and accompanying video demonstrates the typical ultrasound features of HCC. Lesions can be hypo- or hyperechoic with reference to the background liver and often demonstrate a peripheral hypoechoic ring.
Cholangiocarcinoma
Cholangiocarcinoma is an uncommon malignancy with a poor prognosis: the majority of patients will only be suitable for palliative measures [45]. Tumours may be intrahepatic (IHC), proximal extrahepatic (hilar, HC) or distal (DC) and may be multifocal. Surgery offers the only potential cure in patients with localised disease but is associated with significant morbidity and mortality. Accurate preoperative staging is essential to avoid unnecessary morbidity and to plan the surgical approach to treatment. While improvements in cross-sectional imaging have made a great impact in the staging of other GI malignancies, the evaluation of cholangiocarcinoma remains a challenge even to the most experienced clinician.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







