Stage
Definition
Tis
Carcinoma in situ
T1
Tumor invades submucosa
T2
Tumor invades muscularis propria
T3
Tumor invades through muscularis propria into pericolorectal tissues
T4a
Tumor penetrates to surface of visceral peritoneum
T4b
Tumor directly invades or is adherent to other organs/structures
N0
No regional lymph node metastasis
N1
Metastasis in 1–3
N1a
Metastasis in 1 regional lymph node
N1b
Metastasis in 2–3
N1c
Tumor deposit(s) in subserosa, mesentery, or non-peritonealized pericolorectal tissues without regional nodal metastasis
N2
Metastasis in 4+ regional lymph nodes
N2a
Metastasis in 4–6
N2b
Metastasis in 7+
M0
No distant metastasis
M1
Distant metastasis
M1a
Metastasis confined to one organ/site
M1b
Metastases in more than one organ/site
Indications
Rectal Cancer
For decades the mainstay of rectal cancer management has been an abdominal perineal resection (APR). With this as the treatment strategy, nuances of the cancer stage are not as important. However, with the increased focus on sphincter preservation and local excision treatment options with transanal endoscopic microsurgery (TEM) or other endoluminal approaches, rectal cancer staging has come to the forefront. Two issues are of central importance in this regard. First, how far does the lesion penetrate the rectal wall? Second, is there lymph node involvement? While hope exists in the future for a better predictor of nodal disease, at present, the best predictor of the N stage for rectal cancer is the T stage [4–7] (Table 19.2). The depth of invasion therefore is the most important predictor of lymph node metastasis and local recurrence in rectal cancer and is essential knowledge in its management.
Table 19.2
Relationship between T stage and node positivity in rectal cancer
T stage | Node positivity (%) |
|---|---|
T0 | 0 |
T1 | 6–12 |
T2 | 17–22 |
T3 | >60 |
Endorectal ultrasound is used in the initial evaluation of both rectal polyps as well as invasive rectal cancer. If a lesion is not invasive, management consists of local excision. For invasive lesions, ERUS is performed shortly after initial diagnosis of a tumor prior to initiation of neoadjuvant therapy. It is far less accurate to stage a tumor after the start of neoadjuvant therapy, as ultrasound cannot differentiate between tumor and postradiation fibrosis [8]. Additionally, the exam may be prohibitively uncomfortable as the patient’s radiation therapy progresses. For smaller, polypoid tumors, it is fairly common to be asked to evaluate a patient after an attempt at polypectomy by another physician, and this situation is frequently hard to avoid. However, the accuracy of ERUS in this situation may suffer. Following electrothermal injury from snare cautery or biopsy, the accuracy of the study decreases as the resulting inflammation and scar can cause significant artifact and lead to over-staging of the lesion.
ERUS is the best available means to delineate the depth of invasion of a tumor, and this will assist in decisions regarding the use of neoadjuvant chemoradiation prior to any surgical intervention. Although numbers vary from study to study, the overall accuracy of T stage interpretation in experienced hands is approximately 70 %, and this does vary depending on the skill level of the examiner and the T stage [9, 10]. uT3 lesions are the most accurately diagnosed, whereas uT1 lesions are the least accurate. In addition to the depth of penetration of a lesion, ERUS has the ability to visualize lymph nodes in the perirectal fat. Lymph node accuracy is slightly lower at approximately 65 %. Although the accuracy of this information is slightly poorer compared to tumor depth, it does add significant information to the staging process. Unfortunately, the specifics of decision making based on these results are beyond the scope of this chapter. If the tumor is obstructing in nature, it cannot be examined in a transanal fashion and a high-quality MRI is the preferred option.
Incontinence
Endoanal ultrasound (EAUS) is used to evaluate the sphincter mechanism in patients suffering from fecal incontinence. The most common cause of anal sphincter complex injury is obstetrical trauma, in particular third- and fourth-degree perineal injuries. Risk factors for these injuries include forceps deliveries, nulliparity, increasing fetal birth weight, labor length, and performance of a midline episiotomy [11]. Other causes of sphincter injury include iatrogenic injury from previous anorectal surgery (hemorrhoidectomy, fistulotomy, sphincterotomy) and other trauma to the perineum.
Sphincter injuries are very common after vaginal delivery and are frequently occult even in the setting of a perineal tear. EAUS can be used to evaluate for these sphincter defects and is used as a main determinant as to whether someone is a surgical candidate for sphincteroplasty in the treatment of fecal incontinence. EAUS can be used to identify and evaluate both the internal and external anal sphincter. In addition, the puborectalis muscle at the top of the anal canal is seen, as is the superficial external sphincter at the bottom of the anal canal. Pudendal nerve terminal motor latency testing is an adjunct test in addition to ultrasound to evaluate the effectiveness of nerve conduction, as prolonged nerve conduction times can indicate nerve damage as a contributor to fecal incontinence. If the conduction study is abnormal, this may predict poorer outcomes after sphincter reconstructive surgery [12].
Perianal Abscess and Fistula
On occasion, EAUS and ERUS have been used in the diagnosis of perianal abscesses and fistulas. It is generally employed when diagnostic difficulty during an exam under anesthesia is encountered. One can use the technology to identify a fistula tract or internal opening in the anal canal for a presumed perianal fistula, and EAUS can increase the rate of both fistula tract identification and internal opening identification when compared with physical exam alone [13]. The injection of hydrogen peroxide into the fistula tract will cause it to become hyperechoic and has been shown to improve the rate of fistula identification. In the settings of anorectal Crohn’s disease, it can be particularly helpful, as the tracts and abscesses can be quite complex. In addition, it is useful in evaluating rectovaginal and anovaginal fistulas. In this instance, visualized sphincter defects may cause one to consider sphincteroplasty along with an advancement flap procedure as opposed to a simple advancement flap in order to heal an anterior fistula in women.
Anatomy of the Rectum and Anus
The rectum is approximately 12–15 cm in length and descends through the pelvis from the sigmoid colon. There are three semilunar mucosal valves (Houston’s valves) that are viewed endoscopically and must be avoided when entering any instrument. More importantly, the direction of the rectum follows the morphology of the sacrum. After a slight anterior direction in entering the anus, the rectum ascends posteriorly first and then anteriorly to the sacral promontory. When first inserting the rigid sigmoidoscope, the tip is pointed anteriorly at the umbilicus. After inserting it a couple of centimeters to traverse the anal canal, the scope is pointed posteriorly along the sacral hollow and then anteriorly once again. This is essential to understand when entering the rigid sigmoidoscope or ultrasound probe.
The histologic layers of the rectal wall must be understood in order to perform and interpret ERUS. The layers include the mucosa, submucosa, muscularis propria, and perirectal fat. The depth of invasion of a tumor through these layers indicates the T stage of a tumor (Table 19.1).
The anal canal begins at the level of the puborectalis muscle. There are two muscular layers in the canal. The outer muscle begins as the puborectalis, which is really a medial continuation of the levator ani muscles at the pelvic floor. This muscle acts as a sling to control bowel function by creating an angle between the rectum and anal canal. With the relaxation of this muscle, there is posterior straightening of the anorectal junction to allow for defecation. Distal to this is the external sphincter muscle, which is separated into deep, superficial, and subcutaneous layers. This muscle is under voluntary control to aid with continence. Traumatic defects in this muscle can lead to incontinence.
The inner muscular layer of the anal canal is the internal sphincter, which is a continuation of the circular muscle layer of the muscularis propria of the rectum. This is an involuntary muscle that creates a baseline tone in the anal canal for continence of liquid stool and gas. Relaxation of this muscle allows for defecation. Reduced tone in this muscle may lead to fecal soiling or leakage. Lateral to all the muscular layers of the rectum is the ischiorectal fat.
Superficial to the muscle layers of the anal canal is the mucosa and submucosa which contain the hemorrhoidal plexuses. The dentate line is grossly visible and separates two distinct histologic areas of the anus. Above it is the anal transition zone which is a purplish epithelial layer of cuboidal cells which lead to the true columnar epithelium of the rectum. Beneath it is a modified squamous epithelium leading to the true squamous epithelium of the perianal skin. Although histologically and embryologically one may view the dentate line as the end of the rectum, from the surgeon’s perspective, the beginning of the anal canal should be viewed as the anorectal ring. This ring is the most proximal part of the muscular anus at the puborectalis. The importance of this lies in the ability of the surgeon to measure on digital exam the level of a palpable lesion above or below this point so he can both describe the location of a lesion without ambiguity as well as make surgical decisions regarding sphincter preservation surgery. Conversely, some may use the anal verge in describing the location of anorectal lesions. This is a poor substitute, as the length of the anal canal varies significantly, and this value will not be able to guide further management.
Technique
Instrument Setup
The BK Medical ultrasound probe (BK Medical, Herlev, Denmark) is the most widely utilized, and although there are other systems, this section will describe the assembly and use of this instrument (Fig. 19.1a, b). The ultrasound probe rotates a transducer continually to provide a 360° cross-sectional image of the rectum and anus. The setup for ERUS and EAUS is similar; however, a different probe tip is utilized. The probe contains a wire at its base which is attached to the computer and monitor console. A metal shaft is placed over the inner ultrasound shaft and secured at its base. An ultrasound crystal is then inserted at the top of the probe.
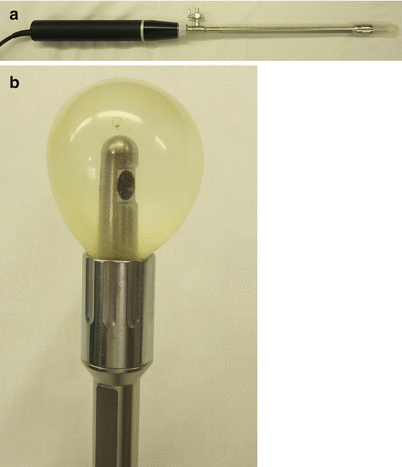

Fig. 19.1
(a) Depicts the standard ultrasound probe with the clear plastic cap used for endoanal ultrasound. (b) Shows a balloon tip inflated with water. The crystal is in the center of the balloon. This tip is used for endorectal ultrasound
The two most common crystals are the 7 and 10 MHz frequency crystals. Newer instruments have a crystal contained within the shaft of the probe that is able to transmit differing frequencies using the console to select them. In general the higher the frequency of the probe, the higher the resolution of the image, but the lower the depth of tissue penetration and what ultimately can be seen. Typically the 7 MHz crystal is used for imaging of the rectum and the 10 MHz crystal is used for imaging of the anus. The reasoning behind this relates to the focal length. Because the focal length of the 7 MHz crystal is between 2 and 5 cm versus only 1 and 4 cm with the 10 MHz crystal, there is a better opportunity to evaluate the perirectal fat for pathologic lymphadenopathy. This being said, one can use increasing frequencies to enhance clarity of the image at the expense of focal distance. It is particularly easy to change between frequencies as desired with newer machine models, which is done by selecting frequencies via the console as opposed to replacing the crystal at the tip of the instrument in older models.
Endorectal Ultrasound
For ERUS, a balloon is placed over the 7 MHz crystal and secured with a rubber ring to the probe. This is further secured with a metal ring. Insufflation of the balloon is necessary to gain contact with the rectal wall, and this is accomplished with sterile water or saline solution. The water is injected through the probe at its side port. Initially, it is necessary to inject water, rotate the probe vertically downward, and withdraw air from the balloon. Water is used as an acoustic window, similar to the “standoff” technique used in abdominal ultrasound (see Chap. 4). Air or bubbles in the fluid of the balloon cause significant artifact and must be removed as fully as possible. This process may need to be repeated several times to ensure no artifacts in the image.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







