Choices in dialysis treatment for infants and children are wide and include the full range of therapies utilized in adult patients. Theoretical considerations of clearance, kinetic modeling, and adequacy of dialysis are equally relevant in pediatric dialysis, although they are less well studied in this population than in adults. There are important technical considerations in performing dialysis on patients whose weights may vary by as much as 50-fold. Furthermore, there are indications for and complications of the dialysis procedure that are unique to children. Finally, chronic care of children receiving dialysis is complex and requires attention to growth and cognitive development, age-appropriate nutritional interventions, consequences of metabolic disturbances, and psychosocial adjustment to achieve the goal of complete rehabilitation.
I. ACUTE DIALYSIS
A. Indications. The indications for acute renal replacement therapy in an infant, child, or adolescent are similar to those in adults and include:
1. Oliguric acute renal failure where optimal nutritional and medical support will require fluid and/or electrolyte removal
2. Volume overload with congestive heart failure, pulmonary edema, or severe hypertension not manageable with diuretics or conservative measures; fluid overload greater than 20% of body weight in the setting of critical illness may be an independent indication
3. Hyperkalemia with electrocardiographic abnormalities
4. Metabolic acidosis that cannot be safely corrected with sodium bicarbonate administration because of risk of sodium or volume overload
5. Symptoms of uremic encephalopathy, with particular attention to seizures
6. Uremic pericarditis
7. Tumor lysis syndrome or severe hyperuricemia complicating chemotherapy for malignancy
8. Progressively rising blood urea nitrogen (BUN) level in a situation where imminent recovery is not anticipated and uremic consequences are likely. The BUN level where concern arises will vary with the age of the child; 35–50 mg/dL (12–18 mmol/L) is potentially dangerous in an infant, whereas 150 mg/dL (54 mmol/L) in an adolescent may necessitate initiation of dialysis.
9. Inborn error of metabolism with severe organic acidemia or hyperammonemia
10. Toxic ingestion. Guidelines for extracorporeal therapy for poisoning are found in Chapter 20.
B. Choice of acute dialysis modality
1. Acute peritoneal dialysis is often used in infants and young children and has several advantages. It does not require sophisticated equipment or technical expertise. One can avoid the need for vascular access, blood priming, and anticoagulation; hemodynamic instability is uncommon. Continuous peritoneal dialysis (PD) provides efficient clearance in small children. It is frequently used as adjunctive therapy to manage fluid overload in infants after cardiac surgery with cardiopulmonary bypass. However, severe hyperammonemia, hyperphosphatemia, or hyperkalemia often require more rapid correction; in such situations, hemodialysis (sometimes in combination with continuous hemo[dia]filtration) may be more appropriate. Furthermore, volume removal by ultrafiltration in PD is often unpredictable and may not be rapid enough in some patients with congestive heart failure or pulmonary edema. Dialysate leakage with risk of peritonitis may limit acute PD.
There are no guidelines as to what constitutes adequate PD in acute renal failure (ARF), and one attempts maximum possible clearance to compensate for catabolic stress, utilizing continuous exchanges. The initial prescription may include hourly exchanges; more frequent exchanges can be performed, although a greater fraction of total time is then spent in filling and draining, rather than in solute exchange. An automated cycler facilitates this process, limiting nursing effort and repeated opening of the catheter. Most cyclers can deliver exchange volumes small enough for infants and young children. When a cycler is unavailable or when fill volumes <150 mL are desirable, a safe alternative is the Dialy-Nate set (Utah Medical Products, Gesco), which allows one to connect a bag of dialysate to a closed circuit, including a buretrol device (in-line sterile graduated cylinder) connected to the patient’s PD catheter, and a drainage line for effluent dialysate attached to a measurement device. The desired volume fills the buretrol and is then infused into the patient; after a defined dwell, the effluent dialysate is drained and measured, and the process is repeated without opening the system. This allows one to perform closed circuit, low-volume, manual continuous PD in infants and very small children.
Exchange volumes may be targeted at 30–50 mL/kg in infants and up to 1,100 mL/m2 in children, but immediately after catheter placement, it is prudent to limit volumes to half or less of this volume to avoid leakage, which predisposes to peritonitis. Hourly exchanges may result in obligate ultrafiltration even when 1.5% dextrose concentration is used, so that parenteral or enteral fluid intake is needed to avoid volume depletion and prolongation of ARF.
2. Acute hemodialysis is performed when rapid solute clearance is of paramount importance or PD is contraindicated because of an intra-abdominal process (including recent abdominal surgery, diaphragmatic hernia, omphalocele, or gastroschisis) or respiratory limitation.
Acute hemodialysis in infants and small children requires experience and technical expertise, as well as size-appropriate dialyzers, blood lines, and vascular catheters. Very small patients may require blood or albumin priming of the hemodialysis circuit. Small patient size allows efficient and rapid solute clearance where appropriate (i.e., ammonia), but must be approached with caution where overly rapid osmolar shifts could precipitate seizures (reportedly more common in children than in adults). Dialyzers are available in a range of sizes for children through older adolescents (Table 37.1); however, the choices in small dialyzers have become fewer, and availability is often limited.
3. Continuous therapies. Continuous renal replacement therapy (CRRT) has been utilized in pediatric patients, ranging from preterm infants to older adolescents. The physiologic principles are unchanged from those in adults (see Chapter 15); because of small patient size, clearance can be extremely efficient, replacing a large fraction of endogenous renal function. Prospective registry data on CRRT in infants and children are becoming available and providing insights into practice variation and determinants of outcome (Ashkenazi, 2013). We recognize fluid overload as an independent risk factor for mortality in children with acute kidney injury (AKI) receiving CRRT and adjust ultrafiltration to address that. CRRT has been successfully combined with extracorporeal membrane oxygenation support even in infants and provides better volume management than free-flow systems. Further, continuous therapies permit better phosphorus clearance than intermittent hemodialysis or PD and are thus frequently employed in the tumor lysis syndrome in children with Burkitt lymphoma or acute lymphoblastic leukemia.
Maintaining vascular access with adequate flow in small vessels can be problematic (Table 37.2) and is often the limiting factor. There are older reports of arteriovenous hemofiltration (CAVH), but most centers have found pump-driven venovenous treatments to perform more reliably and to maintain circuit patency longer. As in acute hemodialysis, the entire circuit volume must be considered and a blood or albumin prime used if the circuit volume is >10% of the patient’s estimated blood volume. The electrolyte concentrations and pH of the blood prime are far from normal values, and many infants will experience hemodynamic instability at initiation of therapy. Zero balance ultrafiltration has been proposed to bring the electrolyte concentrations in the blood prime close to physiologic values, which might avoid instability at initiation (Hackbarth, 2005). Cooling of the blood circuit is a concern in infants; a blood warmer may be used in-line, although with some models this increases the circuit volume. Hemofilters appropriate for pediatric use are listed in Table 37.3. Ultrafiltration is controlled by volumetric pump or automated weighing to avoid errors in replacement fluid, which, if compounded over days of therapy, could be dramatic in a small, anuric patient.
Characteristics of Low-volume Dialyzers Suitable for Pediatric Use | |
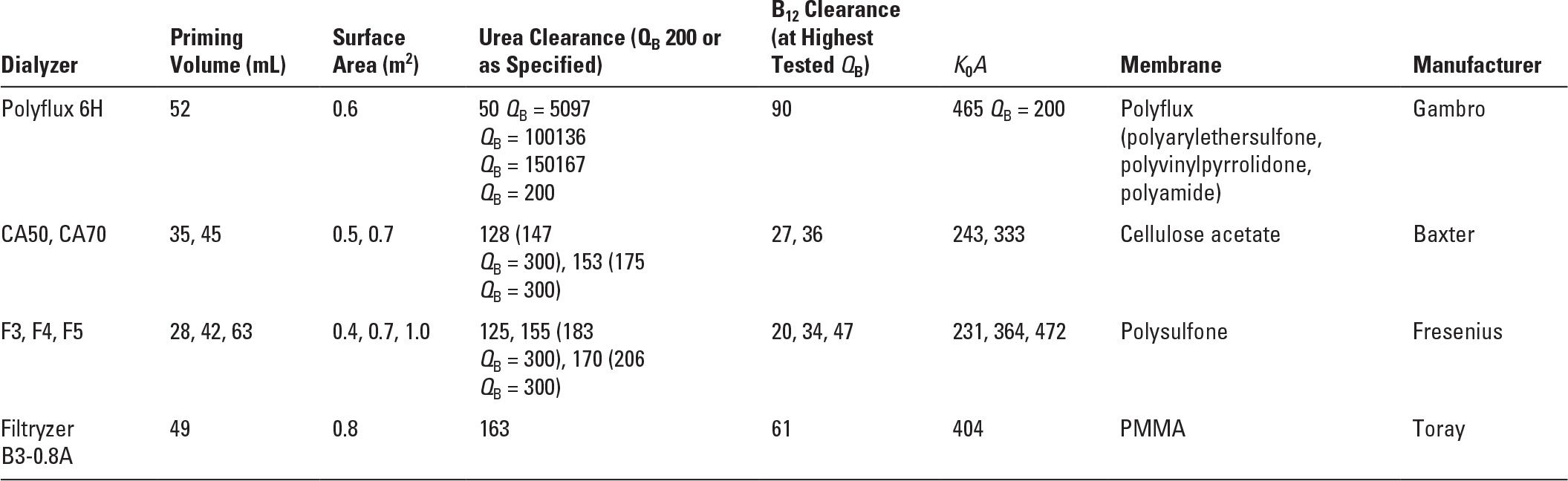
PMMA, polymethylmethacrylate.
Catheters for Use in Pediatric Extracorporeal Renal Replacement Therapy | |
Patient Size | Catheter Size | Access Location |
Neonate | UVC—5.0 F | Umbilicus |
UAC—3.5, 5.0 F | Umbilicus | |
or 5.0 F single lumen | Femoral vein(s) | |
or 6.5, 7.0 F dual lumen | Femoral vein(s) | |
3–15 kg | 6.5, 7.0 F dual lumen | Femoral/subclavian vein |
16–30 kg | 7.0, 9.0 F dual lumen | Femoral/internal jugular/subclavian |
>30 kg | 9.0, 11.5 F dual lumen | Femoral/internal jugular/subclavian |
UVC, umbilical vein catheter; UAC, umbilical artery catheter; F, French gauge.
Currently available machines, including the Gambro Prismaflex (Gambro Lundia AB, Lund, Sweden), the Braun Diapact (B. Braun Medical, Bethlehem, PA), and the NxStage (NxStage Medical Inc., Lawrence, MA), have been used in children, although the NxStage does not permit blood flow rates in a range appropriate for small patients. Several studies have demonstrated the success of CRRT in critically ill infants and children. Ultrafiltration rates in infants and small children may be as low as 5–30 mL/hr without replacement fluid (slow continuous ultrafiltration, SCUF) or as high as 100–600 mL/hr with replacement fluid (C-HF); larger children can tolerate ultrafiltration and replacement rates near those of adults. Commercially available bicarbonate-based dialysate or replacement solution (PrismaSol, PrismaSATE, [Gambro Lundia AB, Lund, Sweden], Accusol, [Baxter Healthcare, Deerfield, IL], Pureflow (NxStage Medical, Inc., Lawrence, MA), Normocarb (Dialysis Solutions Inc., Whitby, ON), or Hemosol BO [Gambro Lundia AB, Lund, Sweden), is the safest choice; errors in local preparation of solutions in hospital pharmacies are well recognized and are no longer appropriate now that standardized solutions are available. Successful circuit anticoagulation has been reported with both heparin and citrate. Since the citrate infusion rate is scaled to circuit blood flow, which is relatively large in infants and small children, citrate accumulation may occur after prolonged therapy, resulting in “citrate lock” or persistently low ionized calcium levels despite calcium infusion. The combination of fixed concentration bicarbonate-containing replacement fluid and citrate anticoagulation may result in metabolic alkalosis after several days of therapy. In reported series, infants <5 kg are more often anticoagulated with heparin. Doses for systemic heparin anticoagulation in infants are larger than those reported for adults, and monitoring the system by activated clotting times (ACTs) is recommended. Circuit life is significantly shorter in pediatric patients run without anticoagulation.
Hemofilters and Sets Appropriate for Pediatric Use | |
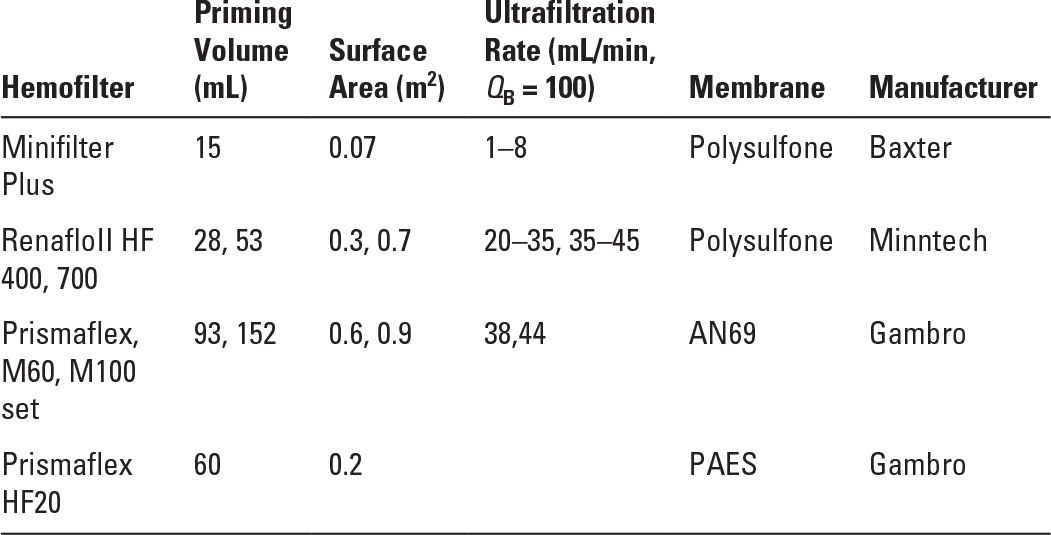
AN69, acrylonitrile and sodium methallyl sulfonate; PAES, polyarylethersulfone.
II. CHRONIC DIALYSIS
A. Indications. Optimal management of chronic kidney disease (CKD) avoids some of the historical indications for initiation of dialysis. Anemia, acidosis, hyperparathyroidism, and growth delay can often be managed medically, so nephrologists must be attuned to subtle indications of uremia, that is, diminished energy (less vigorous play), resumption of napping, anorexia (with absence of expected weight gain), and inattentiveness at school or failure to attain expected developmental milestones, in order to recognize the appropriate time to begin dialysis. There is no consensus as to the specific level of GFR at which dialysis should be started. Symptomatic uremia or metabolic disturbances such as hyperkalemia, hyperphosphatemia, malnutrition, or growth failure that cannot be managed conservatively are agreed upon as indications for initiation of renal replacement therapy. Chronic dialysis is usually an interim measure to allow time to prepare for kidney transplantation.
B. Choice of chronic dialysis modality
1. Chronic PD is often the therapy of choice for pediatric patients. Transperitoneal solute exchange in children appears to be as efficient as in adults. Since peritoneal surface area is correlated with body surface area, small children have a relatively large surface for solute exchange compared with adults, which makes PD an effective modality. Peritoneal equilibration testing (PET) shows that very young children are more likely to fall in the category of high or high-average transport, although this observation appears to be the result of large surface area for transport rather than a difference in peritoneal membrane characteristics and can be corrected for by testing patients at fill volumes of 1,000–1,100 mL/m2. Adolescents and teenagers have PET results more typical of adults. Enhanced glucose absorption will result in relatively rapid attainment of osmotic equilibrium between dialysate and plasma, limiting ultrafiltration on long dwells. Automated forms of PD utilizing short dwells are most commonly used in children to accommodate high average peritoneal transport in young children and to improve treatment adherence in older children.
PD offers additional benefits as a chronic dialysis modality. It is technically simple and avoids the need for chronic vascular access (which is particularly difficult in infants and small children). Blood pressure and volume status may be better controlled with PD than with hemodialysis. Less time is spent in the hospital and in the dialysis unit, with more time spent at school and engaged in other age-appropriate activities. Parents often feel they have greater control over their child’s care when they perform PD.
a. Limitations to peritoneal dialysis. Previous abdominal surgery may result in intra-abdominal adhesions that make PD impossible, particularly repair of complex urogenital anomalies, which are often a cause of end-stage kidney disease (ESKD) in children. However, one can rarely predict whether adhesions will limit therapy, and a trial of PD is usually warranted. The presence of a ventriculoperitoneal shunt was once considered a relative contraindication to PD; however, multicenter data show that dialysis can be performed successfully without ascending infection even in the setting of peritonitis (Dolan, 2013). The presence of a ureterostomy, pyelostomy, or loop ileostomy is not an absolute contraindication to performance of PD, although the risk of exit site infection and peritonitis with urinary organisms is increased.
b. Transplantation in peritoneal dialysis patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





