In dialysis patients, the mineral bone axis is deranged. In attempts to optimize this, a number of drugs are usually given, including phosphorus binders, active vitamin D derivatives, and calcium-receptor-sensitizers. Dietary restrictions are often required to limit the amount of phosphorus absorbed. To understand how to manage the mineral bone disorder (MBD) of chronic kidney disease (CKD), a basic understanding of its pathophysiology is helpful.
I. PATHOPHYSIOLOGY. Three hormones are involved primarily in maintaining mineral bone homeostasis in early CKD: FGF23, calcitriol (also known as 1,25D or 1,25 dihydroxycholecalciferol), and parathyroid hormone (PTH). These hormones interact with the minerals calcium, phosphorus, and, to a lesser extent, magnesium to ensure adequate mineral absorption from the gut, appropriate mineral excretion by the kidney, and optimal conditions in the bone to permit ongoing mineralization and remodeling.
As kidney function declines, there is a progressive loss of the ability to maintain mineral homeostasis and normal bone turnover. The first problem that arises is the need to maintain excretion of phosphorus being ingested from food. The reduced number of functioning nephrons results in an increased phosphorus load being filtered by each nephron. In an attempt to help increase excretion of this added phosphorus load, the levels of the hormone FGF23 (fibroblast growth factor 23) are increased. FGF23, produced by osteocytes, affects the function of renal tubular cells by acting on a Klotho-FGF receptor complex. FGF23 stimulates phosphaturia by decreasing the expression and activity of sodium-phosphate cotransporters in the renal tubules. These transporters normally function to reabsorb filtered phosphorus, and downregulating them increases the per nephron excretion of phosphorus, limiting phosphorus overload.
A second hormone involved in mineral bone homeostasis is calcitriol. Calcitriol is synthesized by the body in a 3-stage process. The first stage occurs in the skin, which, when exposed to ultraviolet light, converts 7-hydroxycholesterol into cholecalciferol (vitamin D3). Cholecalciferol is an inactive steroid prohormone; it becomes slightly active after the steroid ring is hydroxylated in the 25-position by the liver. This so-called “25-D” can then be fully activated by a third step: hydroxylation of the steroid ring at the 1-position. This final hydroxylation step can occur in a variety of tissues locally, but the most important site where 1,25-D is synthesized is in the renal tubules, by an enzyme called 1-α hydroxylase. Another name for 1,25-D is calcitriol. Calcitriol has many actions pertaining to mineral balance. It increases gut calcium and phosphorus absorption, increases calcium reabsorption in the kidney, and suppresses the parathyroid gland from making PTH. Calcitriol also helps mineralize bone.
In early CKD, the levels of calcitriol are reduced. This is thought to occur by two mechanisms: (1) the increased levels of FGF23 induced by the need to increase per nephron phosphorus excretion suppress the 1-α hydroxylase enzyme in the renal tubules, blocking conversion of 25-D to 1,25-D, and (2) there is less conversion of 25-D to 1,25D because of reduced functioning renal mass. The decrease in 1,25D in early CKD may be somewhat compensatory, as slowing calcitriol synthesis results in reduced phosphorus absorption from the gut, and this in turn reduces the phosphorus excretion burden on the dwindling number of nephrons. The reduced serum 1,25-D levels also result in reduction of gut calcium absorption, and higher serum phosphorus levels can result in lower serum calcium levels directly. Thus, some degree of mild hypocalcemia is not uncommonly seen in moderate to advanced CKD.
The third hormone involved in mineral balance is parathyroid hormone. This is a peptide hormone composed of 84 amino acids, with the primary binding to its receptor requiring the presence of the first two amino acids on the N-terminal of the molecule. The main stimulus to PTH secretion is hypocalcemia, which acts on calcium-sensing receptors on the parathyroid gland. One of the main functions of this hormone is to maintain the serum calcium level. PTH does this in a number of ways: (1) PTH decreases the reabsorption of phosphorus in the kidney, increasing urinary phosphorus excretion. This lowers serum phosphorus, which tends to raise the serum calcium; (2) PTH stimulates the activity of the 1-α hydroxylase enzyme in the kidney that converts 25-D to 1,25-D; normally, this results in more calcitriol, and more calcium being absorbed via the gut; and (3) PTH increases the rate of bone turnover, freeing up calcium from bone. Note that PTH and FGF23 both act to increase renal phosphorus excretion, but they have the opposite effects on the kidney enzyme that makes 1,25D. In a feedback loop, secretion of PTH is inhibited by 1,25D acting on calcitriol receptors in the parathyroid gland. This feedback loop can be exploited physiologically and pharmacologically using calcitriol and various analogs of calcitriol to suppress PTH secretion. Finally, PTH secretion is stimulated by high serum phosphorus levels.
As the glomerular filtration rate (GFR) declines and as circulating 1,25-D levels decrease, intestinal calcium and phosphorus absorption are reduced, thus helping to maintain mineral homeostasis. The net effect of the changes in FGF23, calcitriol, and PTH during progressive CKD is maintenance of serum calcium and phosphorus within the normal range until stage 4 or 5 CKD. The low 1,25 D level, the low serum calcium level, and the high serum phosphorus level, however, all act to stimulate PTH secretion and contribute to worsening hyperparathyroidism. With onset of stage 5 CKD and initiation of dialysis, this elegant homeostasis system breaks down, leading to very high FGF23 and PTH levels, uniformly low calcitriol levels, hyperphosphatemia, and low or low normal serum calcium.
These hormonal changes have adverse effects on bone physiology, as described below. Hyperphosphatemia, common in dialysis patients, contributes to the development of hyperparathyroidism and bone disease, and may play a pathological role in the development and progression of cardiovascular disease. High serum phosphorus levels may contribute to impaired bone mineralization and enhance vascular and other tissue calcification. High FGF23 levels, induced by hyperphosphatemia and other factors, have been shown to induce left ventricular hypertrophy in animal models.
II. CONTROL OF HYPERPHOSPHATEMIA. The normal range for serum phosphorus is 2.7 to 4.6 mg/dL (0.9–1.5 mmol/L). In dialysis patients, the KDIGO bone guidelines recommend attempting to maintain predialysis phosphorus in the normal range, based on observational data that better phosphorus control is associated with better outcomes. Also, there are data from animal studies showing that hyperphosphatemia stimulates hyperparathyroidism and promotes vascular calcification. In clinical practice, most physicians and dietitians strive to maintain predialysis phosphorus between 3.0 and 5.5 mg/dL (1.0–1.8 mmol/L).
Hyperphosphatemia occurs in anuric dialysis patients because the amount of phosphorus removed during three dialysis sessions per week is only a fraction of the phosphorus absorbed from the diet. For this reason almost all dialysis patients being dialyzed three times per week and eating a normal diet are required to ingest some form of phosphorus binder along with their food to limit the amount of phosphorus absorbed.
Hypophosphatemia in dialysis patients following a conventional 3-per-week schedule is not the norm and usually is the result of markedly reduced food intake unless there was some error in drawing the blood (e.g., from the dialyzer outlet instead of inlet at the start of dialysis) or unless there was excessive use of binders. Patients with persistent predialysis hypophosphatemia while off binders usually also have low protein intake, and should be counseled to increase dietary protein and phosphorus intake. Use of phosphorus supplements (K Phos Neutral, consisting of 8 mmol (250 mg) phosphorus, 13 mmol sodium, and 1.1 mmol potassium, starting at one tab daily) is indicated if the serum phosphorus remains below 3.0 mg/dL (1.0 mmol/L).
A. Dietary restriction. Restricting phosphorus in the diet to 800 to 1,200 mg per day is the key to controlling serum phosphorus. Inorganic phosphates, added as preservatives and flavor enhancers to processed foods, are absorbed much more readily than phosphorus in natural food (Gutekunst, 2011). Continuing patient education by a knowledgeable dietitian is the best method to establish and maintain proper dietary habits. See Table 36.1 and Appendix B for foods high in phosphorus (Moe, 2011; Gutekunst, 2011). Although the phosphorus content of foods is related to their protein content, phosphorus is more readily absorbed from sources of animal protein than from those of plant protein (Moe, 2011).
B. Removal of phosphorus by dialysis. Hemodialysis typically removes about 800 mg of phosphorus per treatment regardless of predialysis serum levels. High flux dialyzers and dialyzers with larger surface areas, as well as use of hemodiafiltration, can increase phosphorus clearance to a modest degree (Penne, 2010). For hemodialysis, the total weekly time on dialysis is the most important factor affecting phosphorus removal. After the first hour of dialysis, the intradialysis serum phosphorus level tends to stabilize at a low level. This is different than what happens with urea, the levels of which continue to fall as dialysis is prolonged. The maintained intradialysis serum levels of phosphorus cause it to behave somewhat like a middle molecule, where even prolonged dialysis sessions continue to improve phosphorus removal. Dialysis frequency has an additional impact on phosphorus removal because during the initial hour of dialysis, intradialysis serum phosphorus is at a higher level than during the remainder of the treatment. The average patient needs 24–28 hours per week of dialysis to allow a predialysis serum phosphorus level <4.5 mg (1.45 mmol/L) without use of phosphorus binders. Patients undergoing frequent and long nocturnal dialysis with weekly dialysis times greater than 24–28 hours per week typically require addition of phosphorus to the dialysis solution to prevent hypophosphatemia.
Peritoneal dialysis removes approximately 300 mg per day of phosphorus when being treated with a CAPD regimen of four 2 L exchanges per day. This also is far less than the amount of phosphorus absorbed from the diet, and as a result, most peritoneal dialysis patients require the use of binders to control serum phosphorus levels.
Foods Especially High in Phosphorusa | |
Dairy products (milk, yogurt, cheese)
Organ and processed meat
Beans/peas
Nuts/seeds
Whole-grain breads, bran, and cereals
Many soft drinks (particularly colas)
aSee also Appendix B.
C. Residual kidney function. Residual kidney function contributes substantially to phosphorus removal from the body, and patients with urine volumes >500 mL per day typically require substantially lower amounts of phosphorus binders and have lower predialysis serum phosphorus levels than anuric patients (Penne, 2011).
D. Phosphorus binders. Phosphorus binders play an important role in phosphorus control in conjunction with dietary restriction. These agents work by binding phosphorus in the gastrointestinal tract, either by forming an insoluble complex or by binding it into a resin. Despite phosphorus dietary restriction and adequate hemodialysis, approximately 90% of dialysis patients continue to need oral phosphorous binders in an effort to control their phosphorus levels. Other than simply lowering phosphorus, recent observational data have suggested that the use of phosphorus binders may also correlate with longer survival and better nutritional status for patients on maintenance hemodialysis (Lopes, 2012; Cannata-Andia, 2013).
See Table 36.2 for a summary of commonly used phosphorus binding agents. One can think of phosphorus binders in two broad categories, those that contain calcium (calcium carbonate and calcium acetate) and those that do not (sevelamer, lanthanum, magnesium carbonate, sucroferric oxyhydroxide, ferric citrate, and aluminum-containing compounds).
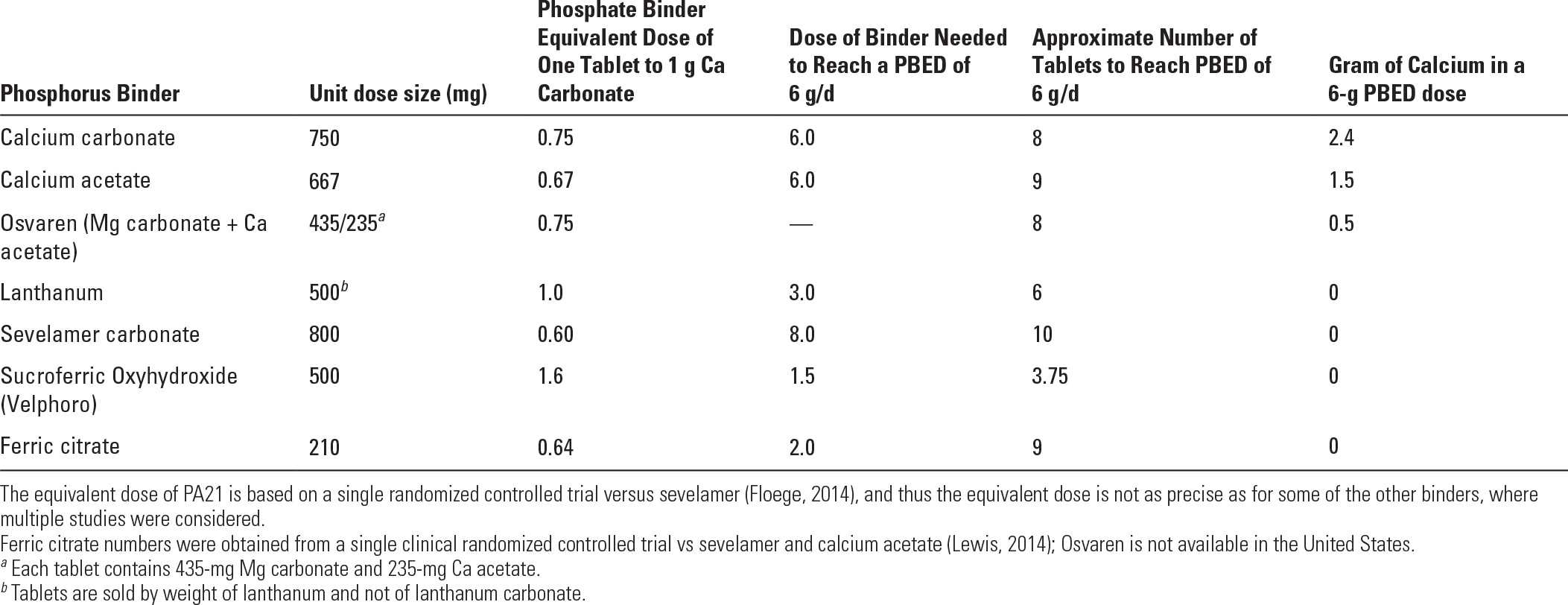
1. Phosphorus binder equivalent dose: Using data from various comparative studies, one can roughly establish an equivalent dose for various binders relative to the phosphorus binding capacity of calcium carbonate (Daugirdas, 2011). This so-called phosphorus binding equivalent dose (PBED) allows one to compare dosages in patients taking multiple binders or different binders. In U.S. patients with minimal residual kidney function being dialyzed according to typical U.S. practices, PBED averages around 6 g per day (Daugirdas, 2012). This means that such patients would need 6 g per day of calcium carbonate to control their serum phosphorus (Table 36.3). The average required PBED is somewhat less, around 4–5 g per day, in smaller patients, those with substantial residual renal function, and is also lower in women versus men, as women tend to eat less phosphorus-rich foods such as meats than men.
2. Calcium load associated with some phosphorus binders. Calcium acetate, on a gram-per-gram basis, is about as effective as calcium carbonate as a phosphorus binder, but calcium acetate contains only 25% calcium by weight, whereas calcium carbonate contains 40% calcium by weight. Thus, attempting to manage a relatively large, anuric patient solely with calcium carbonate would require giving that patient 6.0 g of calcium carbonate per day, and 0.4 × 6.0 = 2.4 g of elemental calcium per day. This is far in excess of the maximum total calcium ingestion recommended by guidelines issued by Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease: Improving Global Outcomes (KDIGO). Using calcium acetate would be associated with a somewhat smaller calcium load of 0.25 × 6.0 = 1.5 g per day. This value is at the upper limit of daily calcium ingestion from both food and binders recommended by KDOQI. For this reason, many patients being managed with calcium-containing phosphorus binders are treated with additional, noncalcium binders.
Selected Phosphorus Binders | |
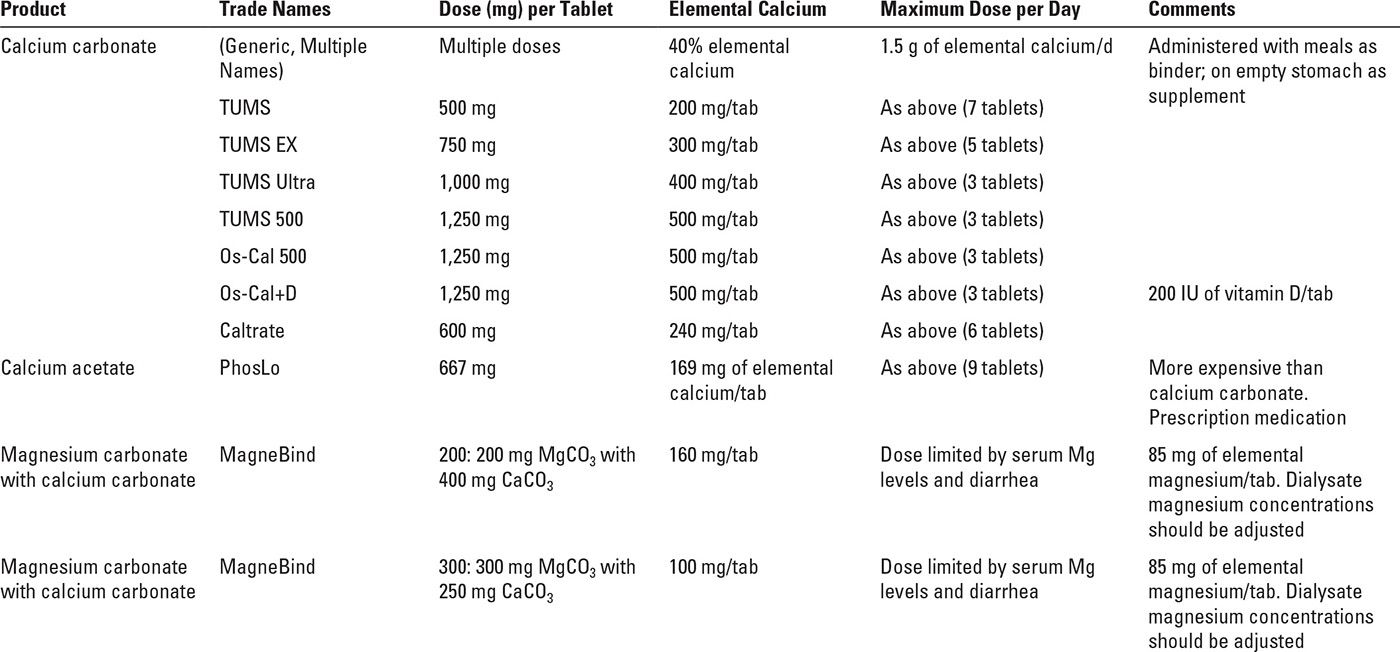
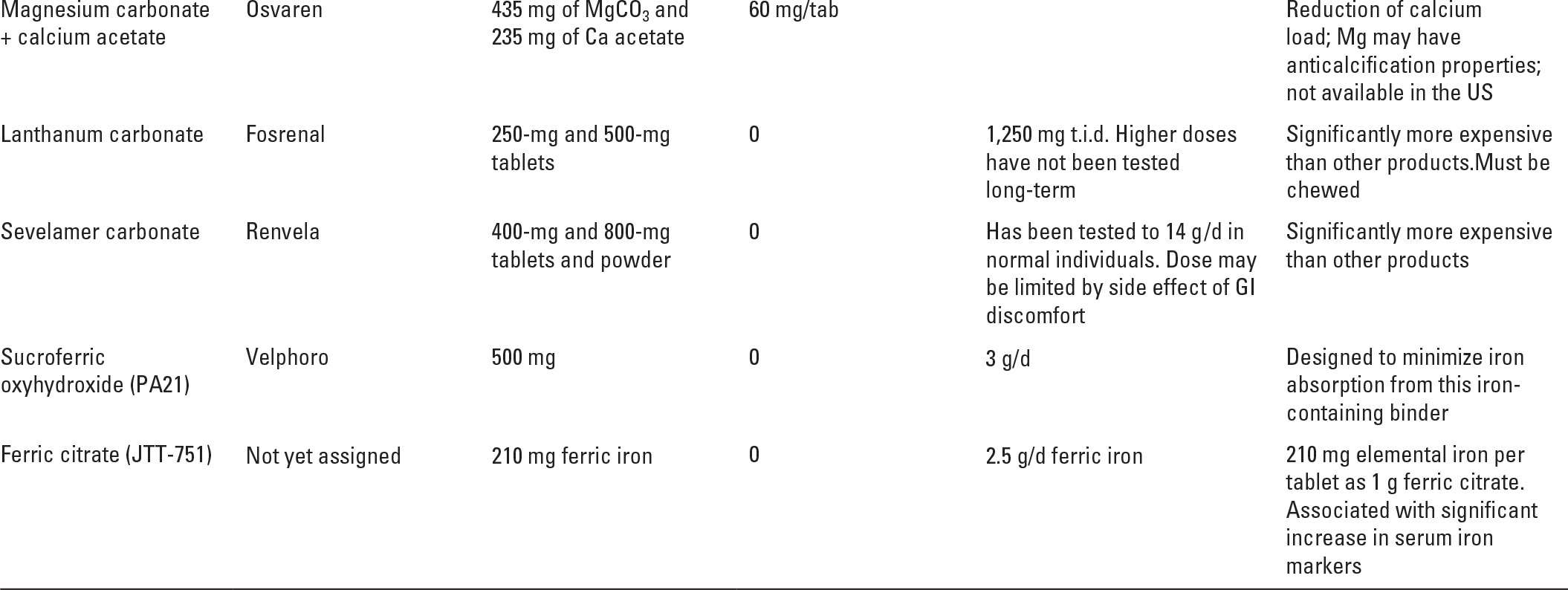
Another strategy is to combine magnesium and calcium compounds to bind phosphorus. In the United States, MagneBind, a mixture of magnesium and calcium carbonate is sold as a dietary supplement and is sometimes used off-label as a phosphorus binder. In Europe, a magnesium/calcium binder made up of magnesium carbonate and calcium acetate (Osvaren) is approved for use as a phosphorus binder based on successful clinical trials (de Francisco, 2010). As shown in Table 36.3, a 6 g per day PBED of Osvaren would be associated with a daily calcium load of only 0.5 g per day. In addition to limiting the amount of calcium absorbed, magnesium-containing binders have at least two potential beneficial effects: (1) magnesium is an anticalcification factor, and it might retard vascular calcification in dialysis patients, although the evidence for this is limited (Spiegel, 2009); (2) mortality tends to be reduced in dialysis patients with higher serum magnesium levels, although it is not clear that magnesium supplementation beyond achievement of physiological serum levels is beneficial. Magnesium overload is something that one needs to watch for in any dialysis patient ingesting sources of magnesium.
A more effective strategy to eliminate the problem of calcium absorption from phosphorus binders is to use one of the newer binders that contain no calcium. Guideline groups recommend avoiding calcium-containing phosphorus binders in patients prone to vascular calcification, or with evidence of vascular calcification; this is hard to do, as the great majority of dialysis patients, especially patients with diabetes, will have evidence of calcification on abdominal X-ray or on visualization of the heart valves.
3. Dosing relative to meals. Phosphorus binders are much more effective when they are ingested with meals, and where the amount of binder given corresponds to the phosphorus load of each meal (Schiller, 1989). Some of the binders require ingestion of multiple pills, while reducing the number of pills by making them larger can make swallowing them more difficult. This has been addressed partially with some binders by making them chewable, or else supplying them as a powder that can be sprinkled over food.
Dosages of Selected Phosphorus Binders Required to Reach a Phosphorus Binder Equivalent Dose (PBED) of 6.0 g per day | |
III. SELECTED PHOSPHORUS BINDING DRUGS
A. Calcium-containing compounds. These agents are often used in the initial management of hyperphosphatemia based on a profile of effective phosphorus binding and low cost. They may also be useful when some degree of calcium supplementation is desired. However, dose titration is limited by KDIGO recommendations that elemental calcium ingestion should generally not exceed 1.5 g per day. In addition, dialysis solution calcium concentration should be limited to 2.25 to 2.5 mEq/L (1.12 to 1.25 mM) to avoid positive calcium balance during dialysis. The coadministration of calcium and active vitamin D preparations predisposes up to 50% of patients to hypercalcemia, and this practice warrants close monitoring (Schaefer, 1992).
1. Calcium Carbonate (40% elemental calcium by weight) is available in a variety of preparations and dose sizes, including TUMS (200 mg of elemental calcium with the regular tablet formulation), Caltrate (240 mg of elemental calcium/tab), and OsCal 500 (500 mg of elemental calcium/tab). A reasonable starting dose is 1–2 tablets with each meal. However, the use of more than 1.5 g of elemental calcium per day exposes patients to excessive calcium loading and the risk of hypercalcemia, and so it is usually impossible to control phosphorus with calcium carbonate alone without very substantially exceeding the maximum recommended targets for calcium ingestion.
Calcium carbonate is available typically as a swallowed tablet, although TUMS does come in chewable formulations. It should be noted that calcium carbonate dissociates best in an acidic environment, and consequently its solubility can be inhibited by medications such as proton pump inhibitors. This agent has the benefit of easy accessibility and low cost. Common side effects include hypercalcemia, constipation, and nausea.
2. Calcium acetate (PhosLo, 25% elemental calcium by weight) is available in 667 mg tablets (169 mg of elemental calcium), and the recommended initial dose is two tablets with each meal. Upward titration may be necessary every 2–3 weeks to establish adequate phosphorus control to a maximum daily dose of 1.5 g of elemental calcium. On a mg calcium carbonate versus mg calcium acetate basis, efficacy of the two drugs as phosphorus binders appears to be similar. However, because calcium acetate is 25% calcium while calcium carbonate is 40% calcium, calcium acetate use is associated with less calcium loading. Still, to achieve a PBED of 6 g per day, use of calcium acetate alone engenders the administration of 1.5 g per day of elemental calcium. Administration is a swallowed tablet, and side effects include hypercalcemia, nausea, and constipation,
B. Sevelamer carbonate (Renvela) is a nonaluminum-, noncalcium-based phosphorus binder that traps phosphorus in the bowel through ion exchange and hydrogen binding. The drug is available in 400- and 800-mg tablets, and granule packets, and should be started at 800–1,600 mg three times per day with meals. It can be titrated upward to a maximum of 13g per day to attain necessary phosphorus control, though this may require a significant pill load and financial burden for the patient. It is recommended to give other drugs 1 hour before or 3 hours after sevelamer administration. The absence of calcium makes sevelamer useful for those predisposed to hypercalcemia and those already at the limit of calcium supplementation. Sevelamer may also have pleiotropic, beneficial anti-inflammatory effects in dialysis patients that are not completely mediated by LDL cholesterol reduction (Rastogi, 2013).
The main side effects of sevelamer are nausea, diarrhea, dyspepsia, and constipation. Sevelamer use may lead to hypocalcemia, which should be treated with supplemental calcium.
C. Lanthanum Carbonate (Fosrenol) became available in the United States in 2005. As a trivalent cation, lanthanum binds phosphorus ionically. It is a noncalcium, nonaluminum-based binder. It is available in 250-, 500-, 750-, and 1,000-mg chewable tablets that may also be crushed. A reasonable starting dose is 500 mg three times per day with upward titration as needed, but not to exceed 1,250 mg three times per day. Very little lanthanum is absorbed, and to date there has been no evidence of toxic accumulation or adverse effects on bone metabolism (Hutchison, 2009). Its main side effects are similar to the other phosphorus binders and are related to GI discomfort. The chewable preparation may be convenient for patients with a large swallowed pill load, but difficult for patients with poor dentition. In a large prospective randomized European multicenter comparator trial, the efficacy of lanthanum carbonate was compared with that of calcium carbonate. Phosphorus control was similar in both groups; however, there was a significantly lower incidence of hypercalcemia in the lanthanum carbonate group, which makes it particularly useful in individuals at risk for hypercalcemia (Hutchison, 2005).
Both lanthanum and sevelamer are notably more expensive than other available phosphorus binders. Although the long-term safety of lanthanum carbonate has been called into question, subsequent reports over 1, 3, and 6 years have demonstrated a satisfactory long-term safety profile (Hutchison, 2009).
D. Magnesium/calcium binders. These include Magnebind (magnesium carbonate plus calcium carbonate), which is sometimes given off-label in the United States, and Osvaren (magnesium carbonate plus calcium acetate), which has been approved for use in dialysis patients in Europe. The potential benefits, as well as minor risks, of giving magnesium in this context have been discussed above.
E. Sucroferric oxyhydroxide (PA21 or Velphoro). PA21 is an iron-containing phosphorus binder that contains no calcium or aluminum. PA21 completed Phase 3 clinical trials in hemodialysis patients and was approved for use as a phosphorus binder in the United States in 2013 (Floege, 2014). It comes as a 500-mg chewable tablet; the starting dose is 1.5 g per day (3 tablets per day with meals) with a suggested maximum dose of 3 g per day. In contrast to ferric citrate, which is another iron-based phosphorus binder described below, Velphoro is associated with only minimal oral iron absorption.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





