Fig. 4.1
Focal nodular hyperplasia, gross. The lesion is lobulated and firm to rubbery. It is well-circumscribed but unencapsulated and often bulges from the liver on cut surface. The background liver is non-cirrhotic
The lesions are usually lobulated and have a firm to rubbery consistency and a lighter color than the surrounding liver, often yellow-tan to light brown. They often bulge from the surface of the liver on cut section. A single or multiple central scars is a characteristic feature of focal nodular hyperplasias and is seen in approximately 62 % of all lesions (Fig. 4.2) [4]. However, the presence of a scar depends considerably on the size of the lesion [19], as central scars are identified in the majority of focal nodular hyperplasias larger than 3 cm, but they are less frequent in smaller lesions (Fig. 4.3).
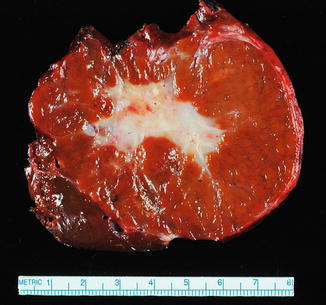
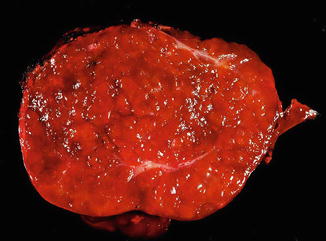

Fig. 4.2
Focal nodular hyperplasia, gross. A prominent central scar is seen

Fig. 4.3
Focal nodular hyperplasia, gross. A central scar was absent in this case
4.1.4 Microscopic Findings
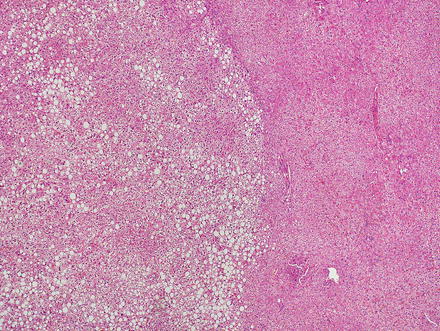
Focal nodular hyperplasias are distinctive, non-encapsulated lesions composed of nodules of benign hepatocytes separated by fibrous septa (Figs. 4.4, 4.5, and 4.6), resembling a focal area of cirrhosis. The fibrous bands often coalesce into a central scar especially in larger lesions. Focal nodular hyperplasias do not contain true portal tracts and the fibrous septa separating the nodules of hepatocytes typically lack native bile ducts. Instead, they contain a bile ductular proliferation that is often associated with mild inflammation typically located at the interface between hepatocytes and fibrous regions (Figs. 4.7 and 4.8). The fibrous scars also can contain abnormal blood vessels (Fig. 4.9). In some cases, the abnormal arteries can show eccentric thickening of the wall. These overall features are typical of larger lesions, but the nodularity may be less developed in smaller lesions, sometimes resembling “bridging fibrosis” rather than cirrhosis (Figs. 4.10 and 4.11).
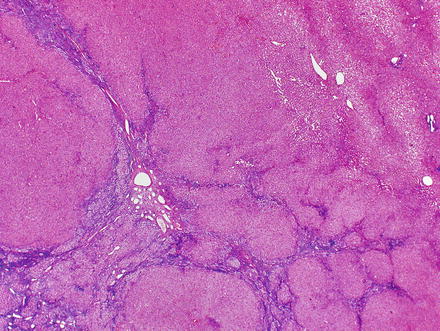
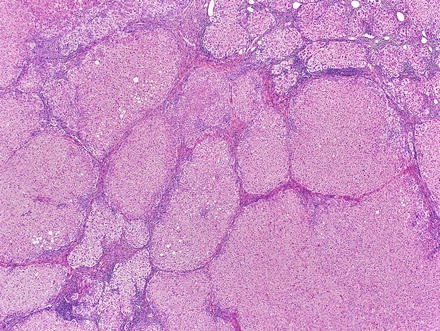
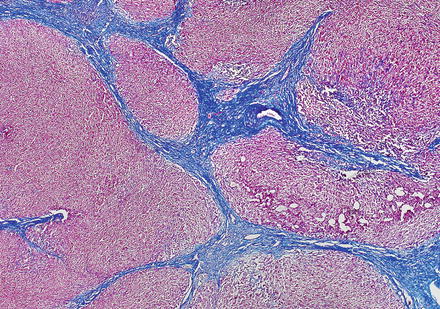
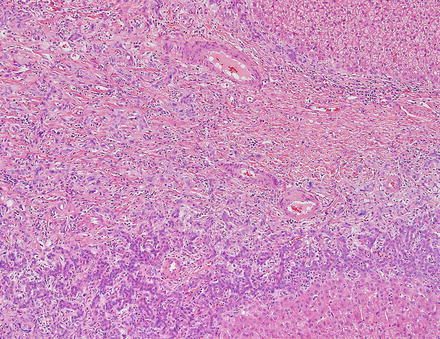
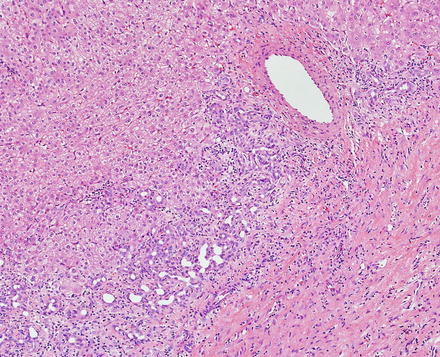
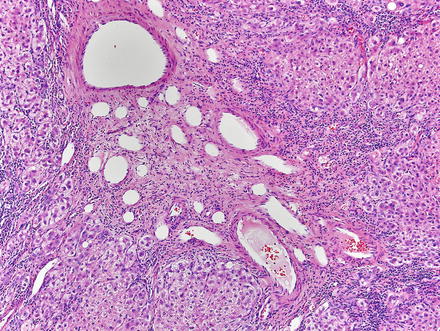
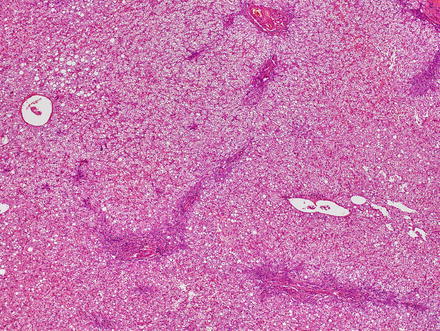
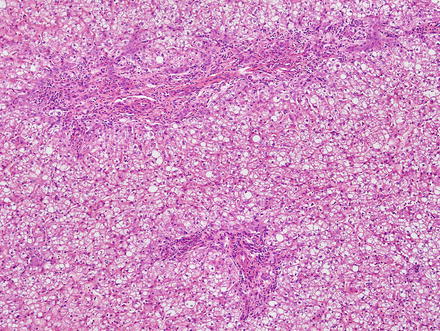

Fig. 4.4
Focal nodular hyperplasia. The lesion (lower left) is distinct from the background liver (upper right) but is not encapsulated

Fig. 4.5
Focal nodular hyperplasia, nodularity. The lesion is composed of nodules of benign hepatocytes separated by fibrous septa

Fig. 4.6
Focal nodular hyperplasia, fibrous bands. A Trichrome stain highlights the fibrosis septa

Fig. 4.7
Focal nodular hyperplasia, ductular proliferation. The fibrous septa lack native bile ducts but show ductular proliferation

Fig. 4.8
Focal nodular hyperplasia, ductular proliferation. A higher power image of the ductular proliferation

Fig. 4.9
Focal nodular hyperplasia, abnormal vessels. A cluster of abnormal vessels is present in a fibrous band at the center of this focal nodular hyperplasia

Fig. 4.10
Focal nodular hyperplasia, early/small lesion. The nodularity is less developed in this small focal nodular hyperplasia and resembles bridging fibrosis more than cirrhosis on low power

Fig. 4.11
Focal nodular hyperplasia, early/small lesion. Although the fibrous septa are smaller and less nodular in this small focal nodular hyperplasia, they still have inflammation, abnormal blood vessels, lack native bile ducts, and show mild bile ductular proliferation
The hepatocytes within the lesion do not demonstrate cytological or architectural atypia. They can, however, demonstrate fatty change, hepatocellular ballooning, and cholate stasis that can be highlighted by copper stains. The latter finding, which typically involves hepatocytes adjacent to the fibrous bands (Figs. 4.12 and 4.13), is due to lack of adequate bile drainage and can be accompanied by Mallory’s hyaline.
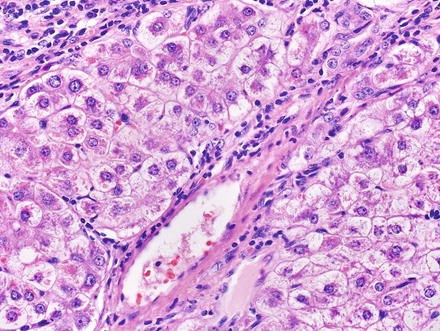
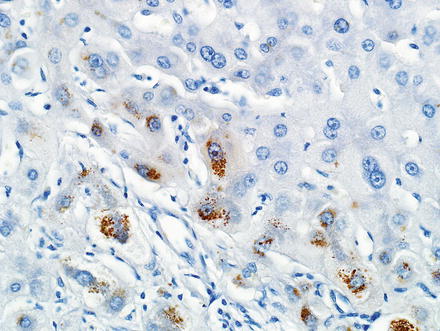

Fig. 4.12
Focal nodular hyperplasia, cholate stasis. Changes of cholate stasis are seen in hepatocytes adjacent to the fibrous bands

Fig. 4.13
Focal nodular hyperplasia, cholate stasis. A rhodanine stain shows copper accumulation in the hepatocytes adjacent to the fibrous bands
The background liver is non-cirrhotic by definition. However, nodules that resemble focal nodular hyperplasia can sometimes occur in cirrhotic livers as a result of vascular shunting; these nodules differ from focal nodular hyperplasia in clinical and biologic characteristics and it is best not to call these focal nodular hyperplasia [20].
4.1.5 Special Stains and Immunohistochemical Findings
Special stains can be helpful in the diagnosis. A reticulin stain demonstrates a normal reticulin meshwork, similar to that of normal liver tissue (Fig. 4.14). Stains for copper demonstrate copper accumulation in hepatocytes, typically located adjacent to the fibrous bands (Fig. 4.13). This occurs due to impaired bile drainage from the lesion [21]. Glutamine synthetase shows a characteristic “map-like” staining pattern in most focal nodular hyperplasias (90 %) (Figs. 4.15 and 4.16), reflecting zonal activation of beta-catenin [22]. There is negative staining in the hepatocytes near the fibrous bands, with positive staining in the more central regions, away from the fibrosis bands. The results of other stains commonly used in evaluating hepatic adenoma are as follows: fatty acid-binding protein (L-FABP) shows retained normal expression, beta-catenin is negative for nuclear expression, serum amyloid-A is usually negative, and C-reactive protein is negative [22].
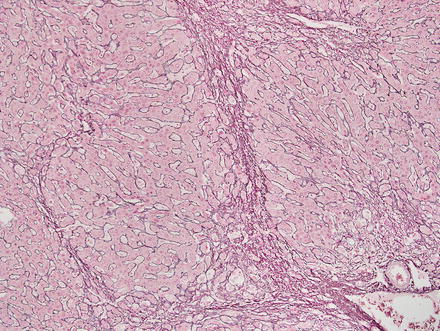
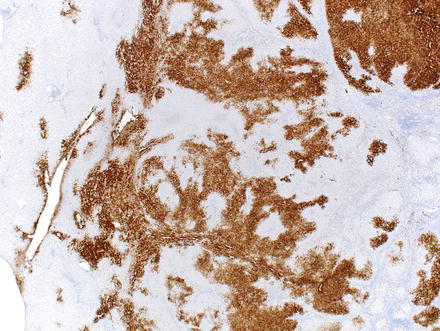
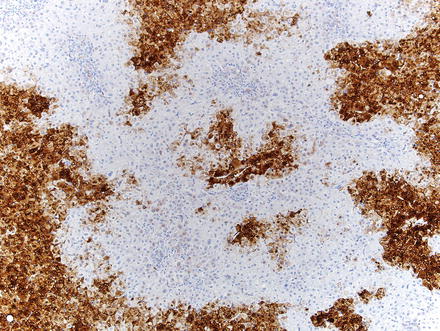

Fig. 4.14
Focal nodular hyperplasia, reticulin. A reticulin stain highlights a normal reticulin meshwork

Fig. 4.15
Focal nodular hyperplasia, glutamine synthetase. A glutamine synthetase immunostain shows a characteristic map-like staining pattern, with interconnecting broad bands of strongly staining hepatocytes, but with no staining of hepatocytes adjacent to the fibrous bands. A small portion of normal liver, with zone 3 perivenular staining of hepatocytes, is seen on the left side of the image

Fig. 4.16
Focal nodular hyperplasia, glutamine synthetase. A higher power image shows that staining typically spares the hepatocytes adjacent to fibrous bands
While a diagnosis of focal nodular hyperplasia in resection specimens is relatively straightforward, making a diagnosis on biopsies can be challenging. The main challenges involve distinguishing focal nodular hyperplasia from hepatic adenoma (especially inflammatory-type) and changes in non-lesional hepatic parenchyma adjacent to a mass lesion (see differential diagnosis below). In part, this reflects sampling issues; multiple passes increase the likelihood of a definite diagnosis. Moreover, there is an increasing trend for only lesions with atypical imaging findings to be referred for biopsy; such cases are less likely to have typical histological features on needle biopsy, which adds to the diagnostic challenge. That said, the diagnosis of focal nodular hyperplasia on needle biopsy is typically possible, especially with the aid of immunohistochemistry (Fig. 4.17). In one study, for instance, a confident diagnosis of focal nodular hyperplasia on needle biopsy was possible based on morphologic grounds alone in about half of the cases, but with the aid of glutamine synthetase staining, the diagnosis became certain in 90 % of cases [23]. Correlation with the imaging studies is also important. Thus, a pathology report might state that the findings are consistent with focal nodular hyperplasia, after correlation with radiologic findings.
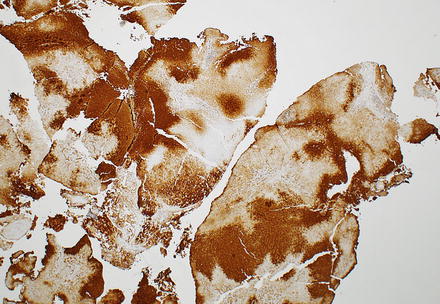

Fig. 4.17
Focal nodular hyperplasia, glutamine synthetase staining in biopsy. The diagnosis was facilitated in this case with a glutamine synthetase immunostain, which highlights a characteristic map-like staining pattern
4.1.6 Differential Diagnosis
The differential diagnosis for focal nodular hyperplasia includes benign reactive changes in non-lesional liver parenchyma, hepatic adenoma, and hepatocellular carcinoma. The differential of hepatic adenoma and focal nodular hyperplasia is discussed in Sect. 4.2.
If the history of a mass lesion is not evident, the differential might include cirrhosis. Some helpful clues include the large abnormal blood vessels with thickened walls and the lack of normal portal tracts. If you’re not sure after careful H&E examination, then glutamine synthetase staining can be very helpful, as the focal nodular hyperplasias will demonstrate the typical map-like staining pattern.
Rarely, mass lesions can have a striking regenerative response at the tumor edges that closely resemble focal nodular hyperplasia. If the tumor itself is not sampled on biopsy, the findings can be mistaken for focal nodular hyperplasia. This is overall a rare finding, but when present the non-neoplastic liver parenchyma adjacent to the mass lesion can show portal expansion with inflammation and fibrosis, bile ductular proliferation, and periportal hepatocellular cholate stasis, all of which can resemble focal nodular hyperplasia. These changes, however, can be distinguished from focal nodular hyperplasia in many cases by the presence of native bile ducts in the portal areas. Glutamine synthetase can also help with this differential diagnosis; focal nodular hyperplasia typically shows a map-like staining pattern with glutamine synthetase, whereas non-lesional liver tissues next to a mass are usually negative for map-like staining and instead stain a rim of zone 3 hepatocytes.
On the other hand, tumors can very rarely induce a vascular shunt that leads to focal nodular hyperplasia-like area at the edges of the mass lesion that are essentially identical to focal nodular hyperplasia. Relatively little has been published about this phenomenon, so the full clinical and pathological correlates are not well-developed, but this can rarely happen with hemangiomas (Fig. 4.18), neuroendocrine carcinomas, and fibrolamellar carcinomas [24]. In these cases, a reactive rim of hepatocytes, featuring histologic features indistinguishable from an otherwise ordinary focal nodular hyperplasia, separates the lesion from the remainder of the liver parenchyma. Distinction from true focal nodular hyperplasia on needle biopsy in these rare cases may not be possible. Nonetheless, in our experience, the imaging findings in these cases are typically inconsistent with focal nodular hyperplasia.


Fig. 4.18
Focal nodular hyperplasia-like lesion developing adjacent to other neoplasms. A lesion identical to focal nodular hyperplasia (left) developed adjacent to this hemangioma
Fibrolamellar carcinomas often have a central scar that radiologically mimics focal nodular hyperplasia [15, 25]. However, the histologic differentiation between focal nodular hyperplasia and fibrolamellar carcinoma is usually straightforward. Fibrolamellar carcinomas have large, eosinophilic cells with cytological atypia, including prominent nucleoli. These features are always absent in focal nodular hyperplasia.
Some focal nodular hyperplasias can also have prominent hepatocellular ballooning and Mallory’s hyaline, findings that may suggest a diagnosis of the steatohepatitic variant of hepatocellular carcinoma. However, focal nodular hyperplasia lacks the cytological and architectural atypia seen in hepatocellular carcinoma. In challenging cases, special stains are helpful. Focal nodular hyperplasias show a preserved reticulin network, while hepatocellular carcinoma shows reticulin loss. Glypican 3 is also helpful when the stain is positive, as positive staining strongly supports a diagnosis of hepatocellular carcinoma. Glypican 3 staining is negative in focal nodular hyperplasias [26]. Finally, a map-like staining pattern with glutamine synthetase favors focal nodular hyperplasia, whereas positive or negative staining is seen in hepatocellular carcinomas.
4.2 Hepatic Adenoma
4.2.1 Definition
A benign neoplasm composed of hepatocytes. Synonyms include hepatocellular adenoma, liver adenoma, and liver cell adenoma.
4.2.2 Demographics and Pathogenesis
Hepatic adenomas are rare, affecting 3–4/100,000 individuals in North America and Europe [27]. Hepatic adenomas are a multifactorial disease in which gender (female), hormones (estrogen and androgens), and genetic predisposition all play important roles [28–30]. The vast majority of hepatic adenomas (approximately 85 %) occur in young to middle-aged women with a history of oral contraceptive use (usually taken for longer than 2 years) [31, 32]. Exogenous androgens and anabolic steroids are also a recognized risk factor in the development of hepatic adenomas [33]. Furthermore, a number of inherited/metabolic disorders are known risk factors for hepatic adenomas, including glycogen storage disease (especially types I and III) and maturity onset diabetes of the young (MODY 3) [22, 34–38]. The adenomas in the setting of glycogen storage disease are more common in males than females [35]. Finally, hepatic adenomas have been reported in individuals with a variety of other conditions including familial adenomatous polyposis, polycystic ovary syndrome, Peutz-Jeghers syndrome, and individuals using the antiepileptic medications carmabazepine and valproate [39, 40]. Clinical associations of hepatic adenomas are summarized in Table 4.1. Pediatric hepatic adenomas are also discussed further in Chap. 13.
Table 4.1
Clinical associations of hepatic adenoma
Associations with hepatic adenoma | |
|---|---|
Established associations | Oral contraceptives |
Exogenous androgens/anabolic steroids | |
Glycogen storage disease | |
Maturity onset diabetes of the young (MODY 3) | |
Isolated reports | Familial adenomatous polyposis |
Galactosemia | |
Tyrosinemia | |
Polycystic ovary syndrome | |
Peutz-Jeghers syndrome | |
Carmabazepine | |
Valproate | |
Portal vein disorders (agenesis, shunts, and hepatoportal sclerosis) | |
Hepatic vein disorders (Budd Chiari) |
4.2.3 Clinical Features
The clinical presentation of hepatic adenoma varies, ranging from asymptomatic mass lesions found during evaluation for other processes, to acute presentation with tumor rupture. In most cases, individuals present with mild nonspecific abdominal pain or discomfort [41]. Hepatic adenomas are often solitary, but recent reports describe multifocality in up to 50 % of patients [41]. When the number of hepatic adenomas exceeds 10, the term “hepatic adenomatosis” is used [42]. Hepatic adenomatosis is seen in 10–25 % of individuals with adenomas and tends to show less association with oral contraceptive use and is more commonly seen in individuals with inherited disorders [40, 43, 44].
Unlike focal nodular hyperplasia, hepatic adenomas carry a risk of bleeding and malignant transformation. Bleeding occurs in 20–25 % of adenomas and can result in tumor rupture and subsequent hemoperitoneum [29, 36, 38, 41, 44]. The risk of malignant transformation into hepatocellular carcinoma is 5–10 %, based on resection specimens [45]. The risk of both bleeding and malignancy increases with tumor size; for instance, in most cases of malignant transformation, the adenomas are 5 cm or more in diameter [41].
Current management guidelines for hepatic adenomas are based mainly on tumor size, patient gender, and to a less degree on histologic subtype (see below, Sect. 4.2.7). Surgical resection is generally recommended for adenomas greater than 5 cm in size. These adenomas are managed more aggressively because of the increased risk of malignancy and bleeding in larger tumors. Hepatic adenomas in males are also generally referred for surgery because of a higher risk for malignancy (up to 50 % risk in some studies). Inflammatory adenomas are often treated by surgery because of modestly increased risk of bleeding. However, much of this increased risk for bleeding appears to be a function of tumor size, and tumors larger than 5 cm would be typically managed by surgery anyway.
Studies also suggest that beta-catenin-activated adenomas have an increased risk for malignancy [30, 36], although not all studies have replicated this finding [41]. The risk of malignancy is not strongly related to the number of lesions, so liver transplantation is not indicated solely on the basis of multiple adenomas [41]. Tumors that do not meet the above mentioned criteria for surgical treatment are managed by observation after discontinuation of oral contraceptive use [41].
4.2.4 Gross Findings
The background liver is non-cirrhotic. Tumors can be solitary or multiple (Figs. 4.19 and 4.20). Although the majority of tumors that undergo resection measure 5–15 cm, some may measure up to 30 cm in diameter [18]. Tumors are well-demarcated, but not encapsulated. Their consistency ranges from soft to firm and the colors range from yellow-tan to brown. The cut surface often has a variegated appearance. Cystic change and hemorrhage can occur, often in the center of the lesion. Although focal scarring in areas of previous necrosis can be seen, prominent central scars of the type usually seen in focal nodular hyperplasia are typically absent.
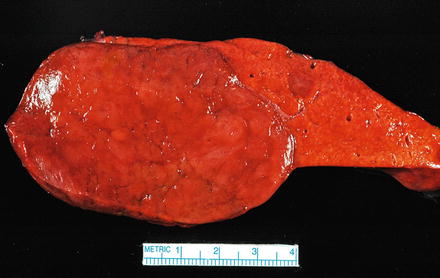


Fig. 4.19
Hepatic adenoma, gross. A solitary hepatic adenoma

Fig. 4.20
Hepatic adenoma, gross. Multiple small hepatic adenomas are seen
4.2.5 Microscopic Findings
Hepatic adenomas can be sharply delimited from the adjacent non-tumor liver (Fig. 4.21) or can blend in with the background liver (Fig. 4.22). In some cases, the adenoma and non-tumoral liver have an irregular border, with intertwined tumor and non-tumor (Figs. 4.23 and 4.24). Hepatic adenomas are typically not encapsulated (see Figs. 4.21, 4.22, and 4.23), but some cases may have a thin fibrous rim of compressed vessels and connective tissue (Fig. 4.25). The background liver usually shows no significant pathology or fibrosis. Exceptions include individuals with glycogen storage disease, in which case some degree of fibrosis can be seen. In addition, individuals with inflammatory type adenomas commonly have an elevated body mass index and their background liver can show fatty change.
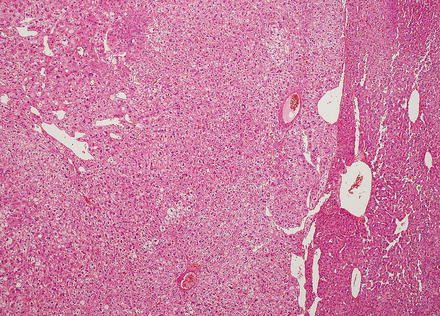
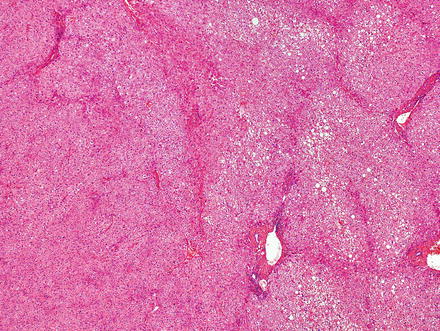
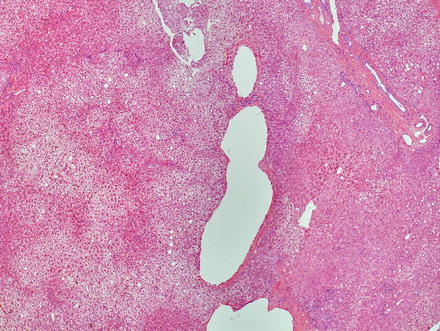

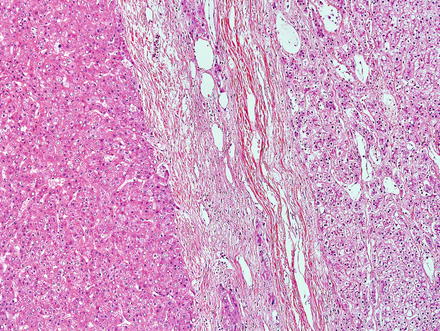

Fig. 4.21
Hepatic adenoma, interface with background liver. This adenoma is sharply demarcated from the liver, but is without a capsule

Fig. 4.22
Hepatic adenoma, interface with background liver. This adenoma (on the left) blends almost imperceptibly with the background liver (on the right). Note that the mild fatty change in the background liver in this case helps visualize the adenoma

Fig. 4.23
Hepatic adenoma, interface with background liver. This adenoma (left side of image) also blends in with the background liver (far right side of image)

Fig. 4.24
Hepatic adenoma, interface with background liver. This is the same image of the adenoma–non-tumor interface show in Fig. 4.23. L-FABP immunostain shows aberrant loss of expression in the adenoma (left), with entrapped tongues of non-neoplastic liver (right) that show preserved expression

Fig. 4.25
Hepatic adenoma, interface with background liver. This adenoma has a thin fibrous capsule
Hepatic adenomas consist of benign hepatocytes; they differ from the background liver by the lack of portal tracts. Instead of portal tracts, aberrant naked arteries are typically scattered throughout the lesion (Fig. 4.26). The hepatocytes within the tumor are fundamentally indistinguishable from normal hepatocytes at high power magnification (Fig. 4.27). They typically have no cytological atypia and essentially no mitotic activity. One exception is androgen-related adenomas, which can demonstrate mild cytological atypia (Fig. 4.28). Hepatic adenomas only rarely have cholestasis (Fig. 4.29) or a pseudoacinar growth pattern (Fig. 4.30); these features are considered atypical and also occur more frequently in hepatic adenomas arising in association with androgen use. Fatty change can occur in hepatic adenomas (Fig. 4.31), but this tends to be characterized by steatosis only, without Mallory hyaline, ballooning, or perisinusoidal fibrosis; finding a true steatohepatitis pattern is atypical for hepatic adenoma and should raise consideration for a steatohepatitic variant of hepatocellular carcinoma. Hepatic adenomas can also demonstrate focal areas of prominent congestion (Fig. 4.32), areas of hemorrhage, and foci of tumor necrosis (Fig. 4.33). Finally, rare adenomas can show areas with striking sinusoidal fibrosis (Fig. 4.34). It is unclear if this represents a degenerative change or the presence of unique genetic changes.
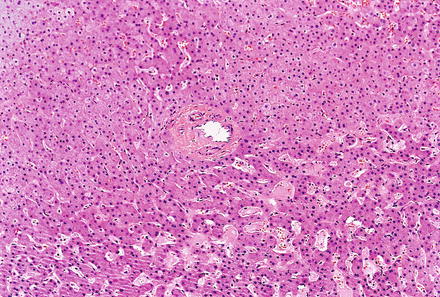
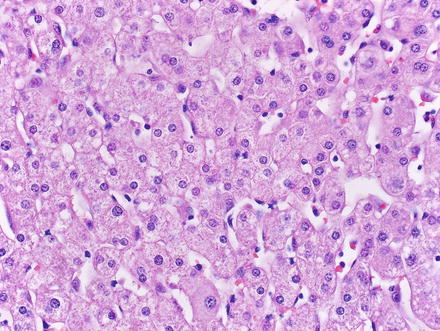
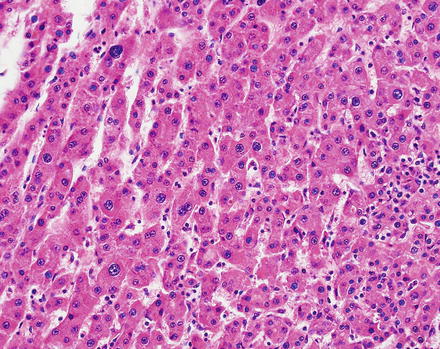
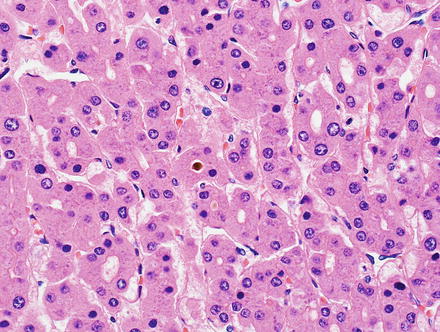
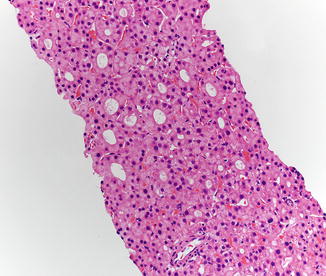
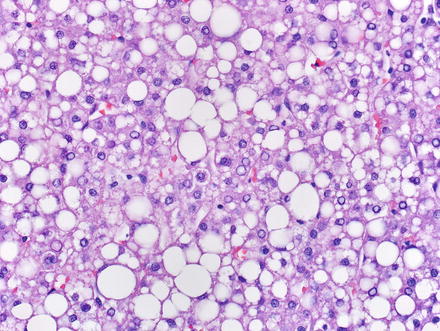
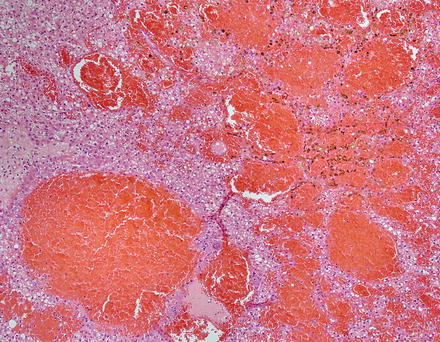
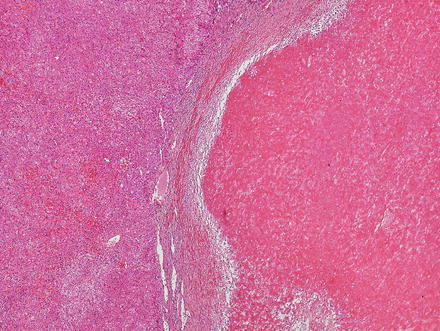
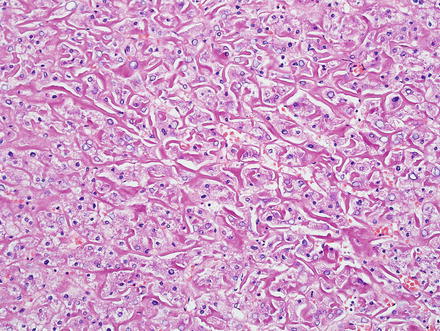

Fig. 4.26
Hepatic adenoma, naked arteries. These aberrant or naked arteries are not accompanied by bile ducts or portal vein branches

Fig. 4.27
Hepatic adenoma, neoplastic hepatocytes. The neoplastic hepatocytes in hepatic adenoma are similar in their appearance to normal hepatocytes; they usually feature no significant cytologic or architectural atypia

Fig. 4.28
Hepatic adenoma, androgen-related. Mild cytological atypia is present

Fig. 4.29
Hepatic adenoma, androgen-related. Cholestasis is seen

Fig. 4.30
Hepatic adenoma, androgen-related. This adenoma also had patchy pseudo-gland formation

Fig. 4.31
Hepatic adenoma, fatty change. Fatty change can be seen in hepatic adenoma, especially in lesions with HNF1α inactivation. Note the absence of steatohepatitic features; such features should raise consideration for hepatocellular carcinoma

Fig. 4.32
Hepatic adenoma, congestion. Marked congestion was focally present in this adenoma

Fig. 4.33
Hepatic adenoma, necrosis. A focal area of ischemic type necrosis was present in the center of this hepatic adenoma

Fig. 4.34
Hepatic adenoma, sinusoidal fibrosis. Rarely, hepatic adenoma can show a heavy deposition of collagen fibers
4.2.6 Immunohistochemical Findings
Hepatic adenomas show an intact reticulin meshwork, similar to that of normal livers (Fig. 4.35). As a caveat, adenomas with fatty change can show occasional foci of reticulin loss that can mimic hepatocellular carcinoma (Fig. 4.36). In addition, there can be areas of reticulin loss adjacent to areas of intratumoral hemorrhage or necrosis (Figs. 4.37 and 4.38). Focal reticulin disruption in this setting should not be interpreted as evidence of malignancy.
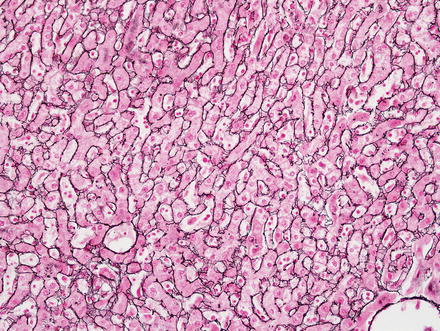
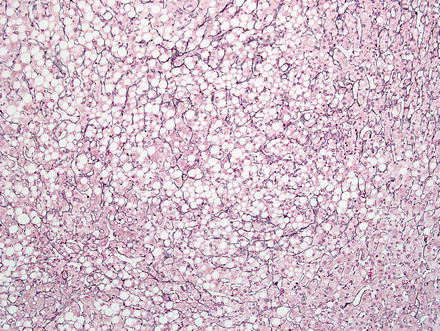
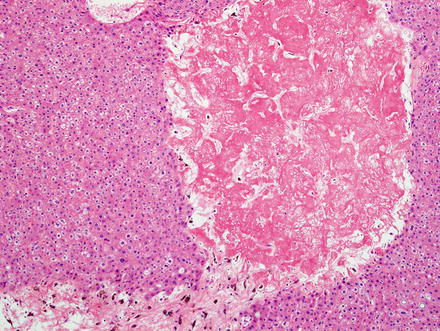
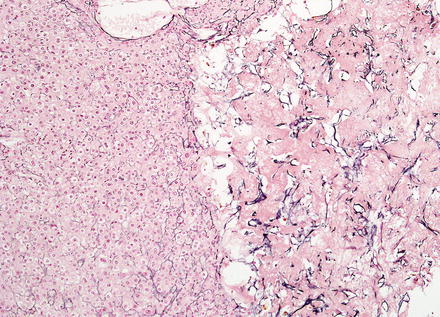

Fig. 4.35
Hepatic adenoma, reticulin. Reticulin stain shows an intact reticulin meshwork

Fig. 4.36
Hepatic adenoma, reticulin. This area of fatty change shows mild loss of reticulin but this does not indicate malignancy

Fig. 4.37
Hepatic adenoma, necrosis. This adenoma showed focal necrosis, but was otherwise histologically typical for adenoma

Fig. 4.38
Hepatic adenoma, necrosis A. reticulin stain in the same area as shown in Fig 4.37 area artifactual reduction of reticulin
Hepatic adenomas are strongly and diffusely positive for HepPar1 and Arginase-1 and are negative for Glypican 3. A CD34 typically shows patchy sinusoidal staining, in contrast to the diffuse sinusoidal staining typical of hepatocellular carcinoma. This marker, however, does not reliably distinguish adenomas from hepatocellular carcinoma in isolation. To illustrate, one study found that 35 % of inflammatory hepatic adenomas showed diffuse CD34 staining [46]. Ki-67 shows a very low proliferative index (<1–2 %) (Fig. 4.39). Inflammatory cells, however, will often show Ki-67 labeling and should be distinguished from tumor cells when evaluating proliferation (Fig. 4.40). One exception is tumor cells adjacent to areas of tumor necrosis, which can focally demonstrate higher proliferative rates (Fig. 4.41).
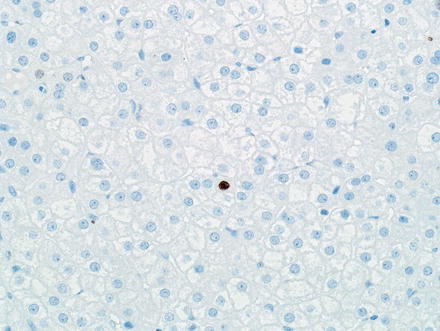

Fig. 4.39
Hepatic adenoma, Ki-67. Hepatic adenomas typically show a very low proliferative index by Ki-67 immunostain

Fig. 4.40
Hepatic adenoma, Ki-67. Inflammatory cells can have a high proliferative index; this should not be misinterpreted as tumor cell labeling. Notice that the inflammatory cells are smaller than the tumor cells and have a sinusoidal location

Fig. 4.41
Hepatic adenoma, Ki-67. An increased proliferative rate was very focally identified adjacent to an area of tumor necrosis
Because biopsy specimens usually provide only partial representation of the entire lesion, they should be interpreted with care. If the clinical history and the histological features are typical, a diagnosis of hepatic adenoma can be comfortably rendered on biopsy. On the other hand, if the clinical findings (e.g., older age or male gender) or histologic features (cytologic atypia, architectural atypia, or focal reticulin loss) are unusual, it is best to use the term “well-differentiated hepatocellular neoplasm,” and explain in a note that the differential diagnosis includes an atypical hepatic adenoma versus a well-differentiated hepatocellular carcinoma. In fact, definitive classification of a small subset of cases cannot be accomplished with certainty on needle biopsy.
4.2.7 Subtypes of Hepatic Adenoma
Hepatic adenomas have been subdivided into four groups based on morphology, molecular findings, and immunohistochemical correlates [30, 36, 44, 47, 48]. Subtyping a hepatic adenoma should only be attempted once the diagnosis of hepatic adenoma has been made; if the diagnosis is uncertain (e.g., adenoma versus carcinoma), immunostains for hepatic adenoma subtyping are not useful. Both resection specimens and biopsies of hepatic adenomas can be successfully classified using immunostains. Although most adenomas are readily classified using this schema, sometimes individual stains can be difficult to interpret and may need to be repeated. The main subtypes of hepatic adenoma are described below and summarized in Table 4.2.
Table 4.2
Adenoma subtypes
Type 1 | Type 2 | Type 3 | Type 4 | |
|---|---|---|---|---|
Genetic abnormality | HNF1α inactivation | Mutations of IL6 signal transducer | Beta-catenin activation | No known mutations |
Fatty change | +/− | +/− | −/+ | −/+ |
Faux portal tracts | − | + | − | − |
Inflammation within lesion | − | + | − | − |
Telangiectasia | −/+ | + | −/+ | −/+ |
Atypia | − | −/+ | +/− | − |
LFABP | Lost | Retained | Retained | Retained |
Beta catenin nuclear staining | − | −/+ | +/− | − |
GS (diffuse strong staining) | − | −/+ | + | − |
CRP/SAA | − | + | − | − |
4.2.7.1 Hepatic Adenomas with HNF1α Inactivation
This subtype of hepatic adenoma is characterized by hepatocyte nuclear factor 1α (HNF1α) inactivation and represents 30–50 % of all hepatic adenomas. Histologically, it often shows steatosis (Figs. 4.42 and 4.43), and by immunostain, shows loss of fatty acid-binding protein (L-FABP) (Fig. 4.44). These tumors usually lack significant inflammation. The steatosis, which is typical but not always present, tends to be moderate to marked and diffuse, but lacks steatohepatitic features. HNF1α-inactived adenomas occur in two distinct settings. First, they can occur by sporadic mutations. Secondly, rare cases are associated with germline mutations in the HNF1α gene, which occurs in the setting of MODY 3. Other clinical correlates of MODY 3 include diabetes before the age of 25 and hepatic adenomatosis (Fig. 4.45). Diffuse glutamine synthetase staining can be seen in about 1 % of HNF1α-inactived adenomas, indicating abnormal beta-catenin activation.