I. INTRODUCTION: TYPES OF VASCULAR ACCESS. Arteriovenous (AV) fistulas and grafts are the commonest forms of vascular access used for maintenance hemodialysis. An AV fistula involves creating an anastomosis between an artery and a native vein, allowing the blood to flow directly from the artery to the vein. Traditionally, the anastomosis is made at the wrist between the radial artery and the cephalic vein, although there are many variations possible, with anastomoses in the snuffbox, in the forearm area, or at the elbow or upper arm. An AV graft is similar, except that the distance between the feeding artery and vein is bridged by a tube made of prosthetic material. The most commonly used bridging material is polytetrafluoroethylene (PTFE) polymer. A third type of access, the cuffed venous catheter, is discussed in the following chapter.
An AV fistula cannot be used immediately as the fistula maturation process generally takes about 6–8 weeks. During the maturation process blood flow through the newly created fistula will gradually increase due to dilatation of both artery and vein. Pressure and flow–induced remodeling (thickening) of the wall of the fistula vein, which is the section where the needles will be inserted, strengthens the fistula and limits tearing and extravasation, while dilatation of the vein facilitates future needle insertion. An AV graft can be used earlier than a fistula, generally within 1–3 weeks after placement.
A well-functioning fistula remains the preferred access compared to a graft due to a fistula’s lower incidence of infection, higher patency rates, and overall better patient survival. However, AV fistulas have their problems as well, one important downside being a poor maturation rate in those with unsuitable blood vessels, including many elderly patients. An AV graft can be a suitable initial choice of access in patients with insufficiently large or poorly distensible blood vessels. With prolonged use, some dilatation of the vein downstream to an AV graft typically occurs, and sometimes this newly enlarged vein segment can then be connected directly to an artery, converting the graft to a fistula.
A. Neointimal hyperplasia. Mechanistically, an AV graft is a less desirable vascular access option than an AV fistula because with a graft there is a higher risk of neointimal hyperplasia. Most commonly, this occurs in the venous segment downstream from the graft–vein anastomosis. Hyperplasia obstructs the lumen of the downstream vein, leading to poor flow in the graft and prolonged bleeding after removal of dialysis needles (due to increased intragraft pressure). Eventually, this leads to graft thrombosis. The cause of accelerated neointimal hyperplasia in AV grafts is thought to be turbulence downstream to the graft–vein anastomosis, and also to a compliance mismatch between the relatively rigid graft and the more flexible vein. Periodic exposure of this vulnerable vein segment to activated blood exiting the dialyzer may accelerate the process, although stenosis can develop downstream to an AV graft even when the graft is left unused.
Although an AV graft is an inferior access option compared to a mature AV fistula, it is superior to a central venous catheter. Patients with either AV fistula or AV graft have less serious infections, lower morbidity, and higher survival rates than patients managed with venous catheters. Recently, some of the poorer results with central venous catheters have been shown to be due to selection bias (venous catheters tend to be used in sicker patients), and the infection risk with venous catheters, especially in elderly patients, has been found to be relatively low (Murea, 2014). Thus, in certain clinical circumstances discussed more thoroughly in the next chapter, the chronic venous catheter remains a useful form of vascular access.
II. GUIDELINES TARGETING INCREASED USE OF AV FISTULA. Guidelines developed by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) and the “Fistula First” initiative (see web references) promote construction of AV fistulas, targeting at least 68% use in prevalent patients on dialysis. Early referral of CKD patients to nephrologists prior to the start of hemodialysis allows more time for construction of an AV access. This avoids the risks of a central vein catheter that is usually required when the patient is referred for dialysis late in the course of chronic kidney disease. Recently “urgent start peritoneal dialysis” has been advocated as an initial treatment method for patients who urgently need dialysis. This allows such patients to be stabilized without subjecting them to a chronic venous catheter. One key factor to increasing AV fistula use is the presence of a dedicated and trained access surgeon functioning as part of a vascular access team.
Over the past decade, since the implementation of the US Government sponsored fistula first breakthrough initiative, the AV fistula rate in the US prevalent hemodialysis patients has increased from 26% to 61%. Many US Centers and European centers achieve much higher percentages (≥90 %). In the United States, however, the central venous catheter use rate has not declined as much as planned, leading to a revision of the initiative goal from “fistula first” to “fistula first and catheter last.”
III. VESSEL PRESERVATION. In patients with progressive CKD who are expected to require dialysis, superficial and deep veins of both arms must be protected, anticipating their possible use for vascular access. Accordingly, one should minimize venipunctures and placement of peripheral infusion lines in the upper extremity, especially in the cephalic and antecubital veins of either arm. The veins on the dorsum of the hand should be used whenever possible. Because of the risk of subsequent central vein stenosis, the subclavian vein should not be cannulated unless absolutely necessary and use of percutaneously inserted central catheter (PICC) lines and midline catheters should be rejected, also. The radial and brachial arteries need to be preserved for future AV access creation, and so cardiac and other endovascular percutaneous interventions should not be done through these arteries. Placement of endovascular leads for a cardiac implantable electronic device (CIED) should be avoided as well, as this can adversely affect patency of the central veins; plus, the long-term risk of infection will be high. Instead, CKD patients who require a pacemaker or similar device should be evaluated for approaches utilizing epicardial as well as subcutaneous lead placement.
A. The American Nephrology Nurses Association “Save the Vein” project. This organization has a website (See Web References) that offers patient-targeted brochures in English or Spanish describing the importance of preserving arm veins. The website also has links to a supplier for patient wristbands bearing the inscription: “Save Veins • No IV / LAB Draws.”
IV. ARTERIOVENOUS ACCESS PLANNING
A. Patient education and timing issues. Patients with a glomerular filtration rate (GFR) of <30 mL/min per 1.73 m2 should be educated about all renal replacement modality options including peritoneal dialysis and renal transplantation. For those choosing hemodialysis, an AV fistula should be placed at least 6 months prior to the planned initiation of dialysis. In patients planning to start peritoneal dialysis, creation of an AV fistula is optional. A backup AV fistula is sometimes created in a peritoneal dialysis patient to avoid the risks associated with central vein catheters when peritoneal dialysis must be stopped for a time; for example, to replace the catheter because of malfunction or severe peritonitis. However, peritonitis rates are much lower now than in the past, so most centers no longer create such backup fistulas. Patients who are planning to receive a live donor kidney in the near future but who need dialysis for a short time can be managed without a permanent AV access. In such patients, short-term use (<6 months) of a cuffed venous catheter for access may be appropriate unless the patient has a contraindication to venous catheter use (such as valvular heart disease, which might predispose to endocarditis).
B. Predicting the need for dialysis. Anticipating the need for dialysis correctly is not always a simple task. Premature creation of an AV access is an unnecessary utilization of resources, and many elderly patients, especially, have been shown to die before needing dialysis. One tool that may help in predicting the need for renal replacement therapy was developed by Tangri (2011, 2013), though their equations predict the risk of developing ESRD over a time window of 3 years. A similar predictive equation based on male US Veterans Affairs patients was developed by Drawz (2013), which predicts the risk of ESRD over a 1-year period.
V. PREOPERATIVE EVALUATION
A. Patient history. A thorough history is required, querying about previous episodes of central vein catheters or intravenous pacemaker/CIED implantation, prior use of PICC lines, and prior vascular surgery. Comorbid conditions such as congestive heart failure, diabetes mellitus, or peripheral vascular disease may limit options for access construction. Patients with severe heart failure may not tolerate the additional cardiac output required to circulate blood through the access. Patients with severe vascular disease due to atherosclerosis or diabetes or patients with extensive damage to their arm veins due to prior needle sticks or failed AV fistula may not have adequate blood vessels to support creation of an AV access, although even in such patients an AV fistula often can be created in the upper extremity using innovative surgical techniques.
B. Physical examination: The presence of all pulses in upper extremity (axillary, brachial, radial, and ulnar) should be evaluated and recorded. The blood pressure in both arms should be measured, and the difference between the arms should be graded as normal if <10 mm Hg, borderline if 10–20 mm Hg, or problematic if >20 mm Hg. The Allen test, which measures collateral flow between the radial and ulnar arteries at the palmar arch, can be either done by physical exam or aided by Doppler (see what follows). The sensitivity of the Allen test can be increased if combined with pulse oximetry (Paul and Feeny, 2003). Details of how to perform the Allen test are given in Table 6.1. The patient should be examined for evidence of previous central or venous catheterization and for signs of trauma or surgery of the arm, chest, or neck, including previous AV access surgery. The presence of arm edema, collateral veins, or differential extremity size should prompt an evaluation of the central veins.
C. Imaging studies. Routine preoperative mapping of the arm to evaluate veins and arteries helps with selection of the most appropriate vein and the best location to create an access. Use of imaging studies has been shown to increase the rate of well-functioning fistula placements.
1. Doppler ultrasonography. Doppler ultrasonography, which can measure flow velocity as well as the inner diameter of the brachial and radial arteries and peripheral veins, should be performed in all patients to identify suitable arteries and veins for access placement. The poor visualization of central veins on Doppler ultrasonography is a limitation of this method. Doppler ultrasonography is best performed in the operating room after regional anesthesia of the arm by nerve block, as the veins tend to dilate postanesthesia administration; under normal circumstances, these veins can be constricted and may not be visualized properly.
Allen Test (Test of Palmar Arch Patency) | |
1. Position the patient so that they are facing you with their arm extended with the palm turned upward
3. Compress both the radial and ulnar arteries at the wrist
3. With the arteries compressed firmly, instruct the patient to create a fist repetitively in order to cause the palm to blanch
4. When the patient’s hand is blanched, release the compression of the ulnar artery and watch the palm to determine if it becomes pink. Then release all compression
5. Repeat steps 2–4 for the radial artery
Interpretation: When color returns to the blanched palm upon release of the arterial compression it indicates arterial patency and reflects upon adequacy of flow. Blanching that persists for ≥5 s after release of the ulnar artery is a positive test for ulnar artery insufficiency. Likewise, blanching that persists for ≥5 s following release of the radial artery is positive for radial artery insufficiency
Modified (corrected) from Beathard GD. A practicioner’s resource guide to physical examination of the vascular access. ESRD Network of Texas. http://www.esrdnet15.org/QI/C5D.pdf.
a. Minimal vein and artery size. Controversy exists about the minimum size of the feeding artery and target vein for a successful fistula. Studies suggest Studies suggest that the minimum vein lumen diameter should be about 2.5 mm for successful surgical anastomosis (Okada and Shenoy, 2014) and minimal arterial diameter should be 2.0 mm. Smaller, “borderline” vessels down to 1.5 mm (for both artery and vein) have been used to create successful fistulas, but this may require an experienced surgeon with skills to operate on such small vessels (Pirozzi, 2010). More important may be the ability of the artery and vein to dilate after anastomosis, to allow an increase in flow.
b. Vein dilation test. During the Doppler study the proximal vein is occluded using a tourniquet and the increase in size is recorded. An average increase in internal diameter of 50% has been associated with successful fistula outcome (Malovrh, 2002).
c. Arterial dilation test. During the Doppler study the pulse contour of the artery is examined. The pulse contour of the artery is normally triphasic, due to high peripheral resistance. The patient is asked to clench the fist for 2 minutes, and then to open the hand; the resulting hyperemic response normally converts the triphasic arterial pulse contour to a biphasic pattern in patients capable of a healthy arterial dilation.
d. Mapping. The cephalic and ulnar venous systems should also be evaluated for continuity and absence of strictures. Some surgeons perform venous mapping with a proximal tourniquet in place to distend and better identify veins suitable for AV fistula construction.
2. Venography. Venography should be reserved for evaluating the central veins, especially in patients with a history of transvenous placement of a pacemaker, physical findings of upper extremity edema, collateral veins around the shoulder or on the chest wall, and/or unequal extremity size. If venography is performed, 30 mL or less of nonionic, low osmolality contrast, diluted 1:4, should be used to avoid nephrotoxicity. Full-strength contrast is usually not required for venography. Venography alone does not help evaluate the arterial tree.
3. Arteriography. Arteriography is indicated when pulses in the desired access location are markedly diminished or absent or there is a >20 mm Hg difference in mean arterial pressure between the two arms.
VI. POSSIBLE LOCATIONS FOR UPPER EXTREMITY AV FISTULAS. For an overview, see Okada and Shenoy (2014) (Table 6.2).
A. Arm fistula locations. An AV fistula can be categorized as conventional or transposed, depending on its connection to the arterial and venous circulations. A conventional AV fistula is created by connecting a superficial artery and vein and generally does not require extensive mobilization of the vessels. A transposed AV fistula utilizes deeper veins and requires extensive mobilization of the vein into a subcutaneous tunnel for easy needle access. Compared to conventional AV fistulas, transposed AV fistulas are technically more challenging to create and require greater healing time. Generally, conventional AV fistulas are created as a single-stage surgical procedure, whereas creation of a transposed AV fistula can be either a one-stage or two-stage procedure.
At least nine potential sites for AV fistula can be used in the upper extremity (Table 6.2). The anatomical snuffbox fistula is the distal variant of the radiocephalic fistula created between the tendons of extensor policis longus and brevis. The classic wrist radiocephalic or Brescia–Cimino fistula (Fig. 6.1) placed in the nondominant arm is the preferred access. Other forearm AV fistulas, such as the ulnar artery–basilic vein fistula, should be considered when a radiocephalic fistula is not an option. Before considering an upper arm site, several other transposed forearm sites should be evaluated; for example, the forearm cephalic vein to proximal radial artery or brachial artery, and the transposed forearm basilic vein to radial artery or brachial artery. If creating a forearm fistula is not possible, which happens not uncommonly in diabetic or elderly patients with atherosclerosis, then an upper arm brachial artery–cephalic vein fistula (Fig. 6.2), or a transposed basilic vein–brachial artery fistula (Fig. 6.3) are potential options. Less commonly used options are the Gracz fistula (which uses a perforating vein that arterializes both upper arm cephalic and basilic veins) and the brachial bidirectional cephalic fistula (which arterializes both forearm and upper arm cephalic veins). When a perforating vein fistula is used, it has been suggested that the original surgical procedure be modified (Konner, 1999). When all sites in the nondominant arm have been exhausted, then the dominant arm can be used.
Sites for AV Fistula Creation in the Upper Extremity | |
Conventional
Snuffbox (distal-most site)
Radiocephalic or Brescia–Cimino (radial artery to forearm cephalic vein at the wrist)
Ulnar artery to forearm basilic vein (uncommon)
Brachial artery to upper arm cephalic vein (at the elbow)
Transposed
Forearm basilic vein to radial artery at the wrist
Forearm basilic vein to the brachial artery (loop configuration)
Forearm cephalic vein to the brachial artery (loop configuration)
Transposed basilic vein in the upper arm to the brachial artery
Perforating veins in the proximal forearm to proximal radial artery (Konner modification of the Gracz fistula)
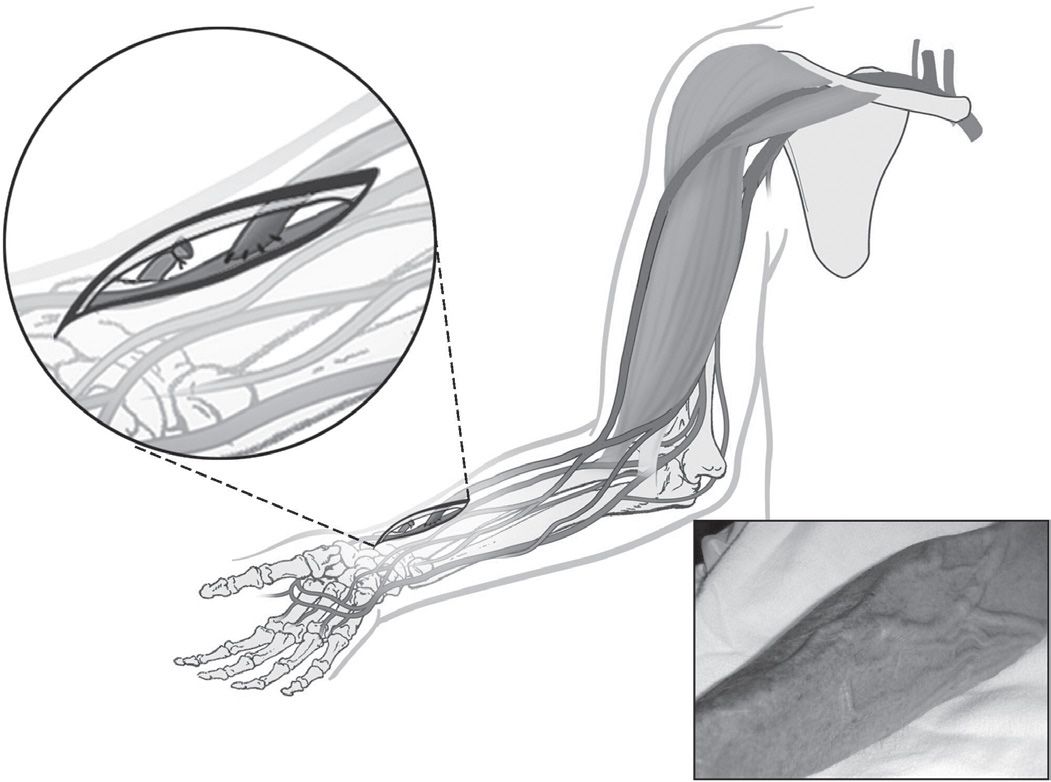
FIGURE 6.1 Radiocephalic AV fistula. (Reprinted with permission from Atlas of Dialysis Vascular Access—http://www.fistulafirst.org.)
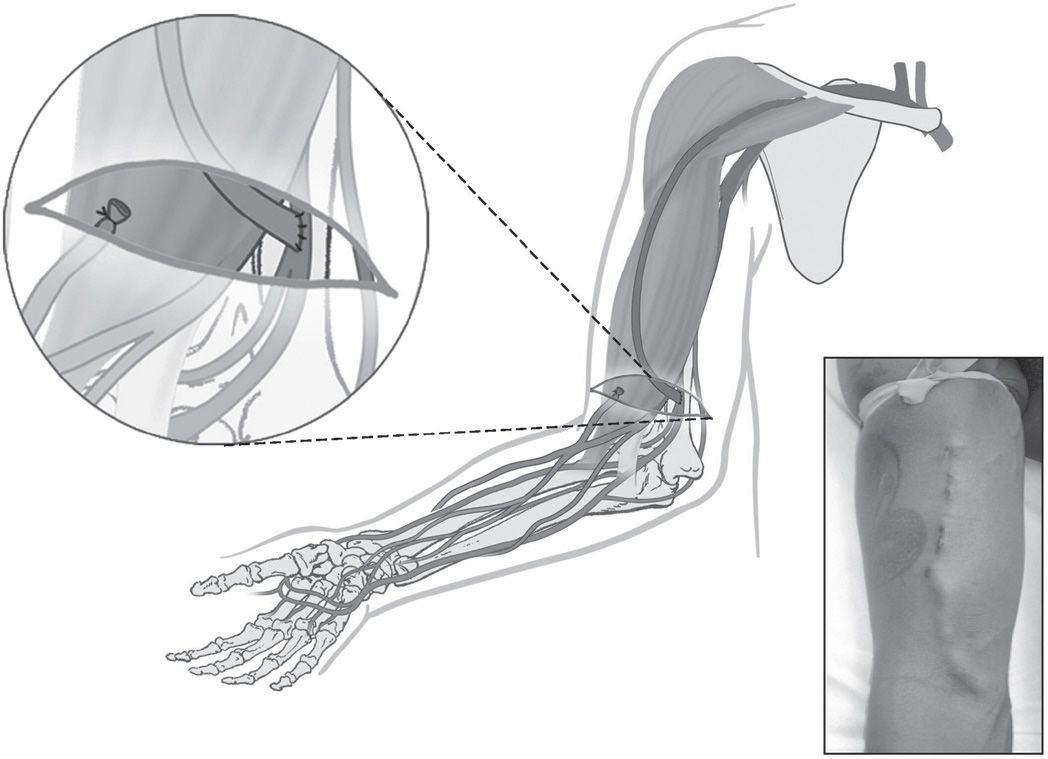
FIGURE 6.2 Brachiocephalic AV fistula. (Reprinted with permission from Atlas of Dialysis Vascular Access—http://www.fistulafirst.org.)
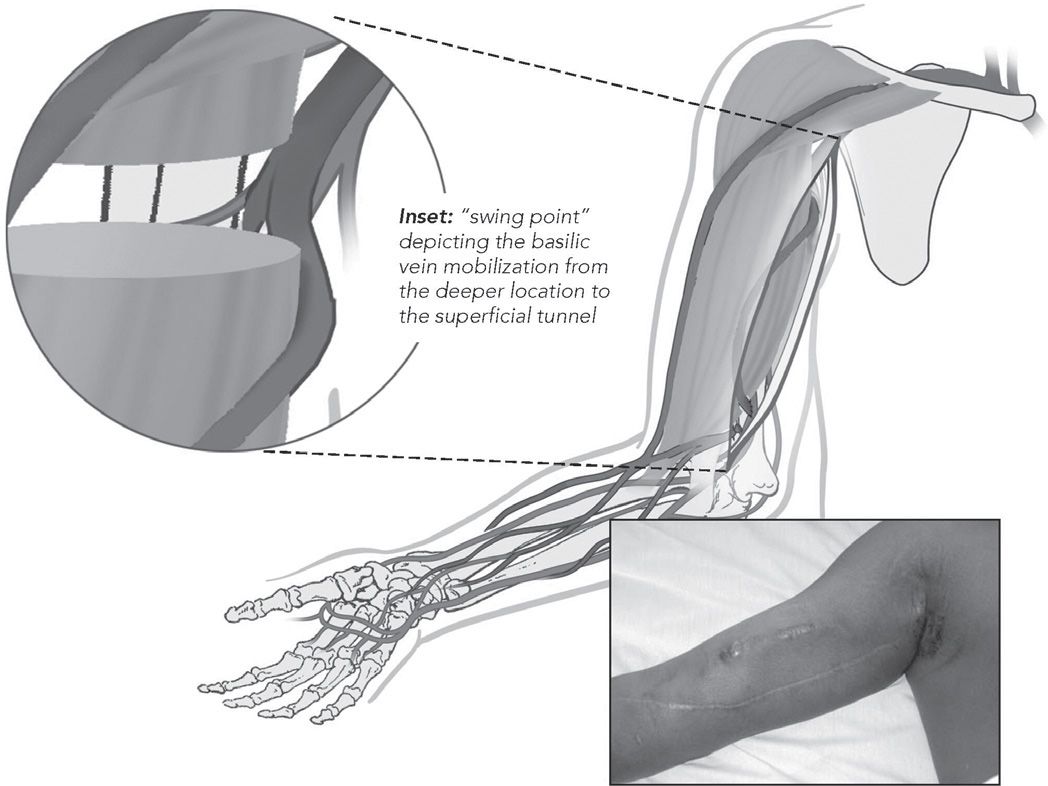
FIGURE 6.3 Transposed basilic vein to brachial artery AV fistula. (Reprinted with permission from Atlas of Dialysis Vascular Access—http://www.fistulafirst.org.)
1. Initial selection of elbow perforating vein fistula for patients who are elderly or with comorbidities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





