Fig. 7.1
Hypothalamus-pituitary-gonadal axis. (Reprinted from Zavos, P. (2009). Male Infertility. Retrieved from http://www.homefertility.com/mi.html. With permission from Prof. Dr. Panayiotis Zavos.)
The onset of normal puberty varies between children, but changes typically associated with normal development can be seen between the ages of 9–13.5 years. The mean age in western countries is 11.5 years [5]. Recent studies have suggested that puberty is occurring earlier in girls and that similarly there may be earlier genital growth in boys [6, 7]. However, these studies have been criticized for their methodology, causing concerns about the conclusions of these studies. Other investigators have found no evidence for earlier pubertal development [8, 9], leaving this question virtually unanswered.
Tanner staging defines five stages of genital and pubic hair development (Fig. 7.2). Signs of puberty in the male child include increases in testicular volume and penile girth and length. Testicular volume increases from 1–2 to 3–8 mL and finally to 20–30 mL in adulthood. As testicular volume increases, testosterone levels rise. When testicular volume reaches 4 mL, plasma testosterone increases from less than 0.1 ng/mL to over 6–7 mg of testosterone being secreted each day [10]. Increases in testosterone, stimulates penile growth. Penile size is usually measured in the flaccid stretched state. While penile size varies, the average length in early development measures 6.7 cm, which increases to 12.4 + 2.7 cm in the adult white male [11]. Other important aspects of puberty include growth of muscle mass, decrease in body fat and increase in bone mineral content, and skeletal muscle maturation [12].
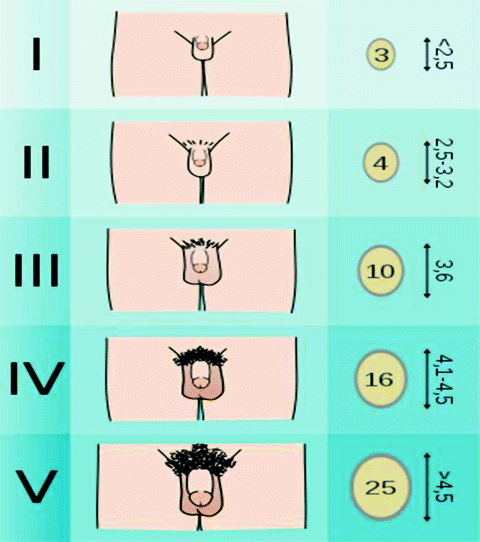

Fig. 7.2
Male tanner stage. (Reprinted from Tanner Scale. http://en.wikipedia.org/wiki/File:Tanner_scale-male.svg. Last Accessed April 24th, 2012.)
Delayed Puberty
When expected milestones in pubertal development are not met, this raises concerns for pubertal delay. Typically, the first step after excluding obvious infirmities is a full endocrinology evaluation. While most parents and children often seek out medical attention earlier, delayed puberty is diagnosed when a male child does not undergo full pubertal development by age of 16 or 17 [13].
One of the most common reasons for delay in normal pubertal development is termed CDGP or transient delayed puberty. Often, families report a known “late bloomer” in the family tree. Parents seek treatment due to delayed growth and delay in the tempo of puberty coupled with delayed psychosocial development as it relates to the child’s peers [14]. While physical and psychosocial development does not necessarily represent chronologic age, these boys are appropriately developed for their bone age. This means that bone age is delayed behind chronological age and the developmental milestones are reached only at a normal bone age. The social implications of delayed puberty for these boys are daunting. The Oakland Growth study followed 16 early and 16 late-maturing males from age of 11 through adulthood. This study found that peers considered delayed males less attractive, more talkative, and less mature. The affected male child describes feelings of inadequacy and rejection through adolescence and into adulthood [15]. A second large study conducted by the National Health Examinations Survey found that teachers usually rated the males affected with delayed puberty as less intelligent. These children had lower intellectual test scores and had lower parental expectations of academic achievement [16]. In addition to the psychosocial issues, there are also physical impediments to delayed puberty. Studies have shown that without treatment, these children do not reach predicted adult height and bone mineral density may be diminished [17, 18]. Androgen therapy in children with CDGP can have beneficial effects on bone mass, body composition, and important psychological aspects. Testosterone is used short-term until the HPG axis catches up and allows for normal spontaneous pubertal development.
Primary or secondary hypogonadism can also cause delayed puberty. This is a permanent form of hypogonadism and requires lifelong androgen replacement therapy. The absence of hormones may be due to hypogonadotropic hypogonadism. In hypogonadotropic hypogonadism, there is insufficient pulsatile GnRH secretion and subsequently a LH/FSH deficiency. The etiology of hypogonadotropic hypogonadism can include tumors, inflammatory processes, trauma, genetic conditions, and developmental defects. Children that present with neurologic signs, anosmia (associated with Kallmann syndrome), headaches, or visual disturbances should be evaluated more thoroughly searching for a central nervous system lesion and have a full hormonal profile. Other causes include anorexia nervosa, malnutrition, and very competitive athletic training. Investigators theorize that in these conditions, an increase in cortisol releasing factor increases beta-endorphins and inhibits GnRH. Increased stress inhibits the HPG axis, resulting in delayed puberty in these males [19]. It can be difficult at times to differentiate between CDGP and permanent hypogonadotropic hypogonadism. However, most experts do not support special testing if the only complaint is delay in pubertal development. If needed, these entities can usually be distinguished once androgen therapy is initiated [1]. Children with permanent hypogonadism have a hypothalamic/pituitary gonadotropin deficiency and while GnRH can be administered, the use for pulsatile administration is somewhat impractical. Testosterone supplementation is a simple solution, and these males will require lifelong androgen therapy, since the normal HPG axis is nonfunctional.
Another rare cause of permanent hypogonadism is impaired secretion of gonadal steroids leading to decreased negative feedback of the hypothalamus. In this case, there is low plasma testosterone in the presence of high plasma LH and FSH levels. A common genetic abnormality associated with hypergonadotropic hypogonadism is Klinefelter syndrome, in which case a 47,XXY karyotype or a mosaic 46,XY/47,XXY is most common [20]. In infancy and childhood, the symptoms associated with Klinefelter syndrome are often nonspecific and the diagnosis can be missed. During adolescence, puberty often starts normally, corresponding to the patient’s peer group, with genital enlargement and pubic hair growth. However, while the testes start to grow, they typically skip growing at 6 mL, leaving a discordance between the degree of sexual development and the size of the testes which often leads to the diagnosis [21]. In adulthood, small testes and a bivariate testosterone⁄LH evaluation outside the normal reference range is a tell-tale sign of Klinefelter syndrome. As the Klinefelter patient moves through adolescence and into adulthood, frequent plasma testosterone and LH monitoring is necessary. In some cases, testosterone replacement is considered when the LH is >2.5 SD above the mean value of 2–14 U/L or when the plasma testosterone is less than 84–480 ng/dL [22]. In many cases, testosterone replacement may be unnecessary, unless an infertility evaluation is initiated. Figure 7.3 lists many of the causes of delayed puberty in the male adolescent.
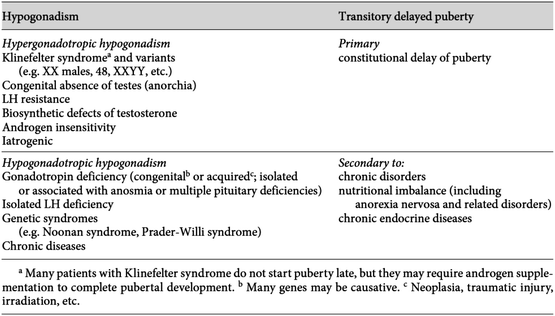

Fig. 7.3
Causes of delayed puberty in males. (Based on data from Bertelloni et al. [26], Kaplowitz, P.B., Delayed puberty. Pediatr Rev, 2010. 31(5): p. 189–95, Leichtnam, M.L., et al., Testosterone hormone replacement therapy: state-of-the-art and emerging technologies. Pharm Res, 2006. 23(6): p. 1117–32.)
Androgen Replacement
The primary goals of androgen replacement therapy in the adolescent male is to promote the linear growth, secondary sexual characteristics, accrual of adequate bone mineral content, and acquisition of normal muscle mass [1, 21]. Secondary goals include normal psychosocial function. In order to obtain these goals, the physician needs to recognize the tenets of testosterone replacement; first of which is to replace testosterone only in those males who are hypogonadal and to insure that the testosterone level is brought to a normal physiologic state. These goals should be obtained regardless of the etiology of hypogonadism. Testosterone replacement must be safe and effective with minimal side effects. While many forms of testosterone replacement have been clinically used in the adult population and are efficacious, these regimens cannot be applied to the adolescent, since a full adult dose of testosterone is not required to initiate or maintain puberty in this age group.
Treatment
Testosterone was first synthesized in the 1930s. The original oral form of T underwent rapid liver metabolism and therefore, was not useful in long-term management of hypogonadism. Other forms were subsequently introduced, but due to hepatotoxicity, were deemed dangerous for human use. The most successful forms of therapy have been the esters (short and long acting). These compounds can be injected and are lipophilic and very durable. More recently, the addition of transdermal forms of testosterone and pellets have made administration of testosterone more convenient for the affected patient. Figure 7.4 gives a brief description of the different forms of testosterone in clinical practice. Note that not all forms of T are available in the US. Several studies have evaluated different testosterone formulations in male adolescents [23–26]. Testosterone enanthate is the gold standard, as it increases muscle mass, accelerates linear bone growth, and induces the appearance of secondary sexual characteristics. This allows for accomplishing the goals of androgen therapy in adolescents [27–29]. Prior to the initiation of androgen replacement therapy, the child presenting with delayed puberty should undergo a complete history and physical examination. Any additional abnormalities need to be addressed first. A complete bone age evaluation including bone density scans of lumbar spine and femoral heads is obtained. When the timing for the therapy is appropriate, testosterone enanthate or cypionate is initiated at a starting dose of 50–100 mg IM every 4 weeks for 3 months. At the 3-month mark, most patients will notice increases in appetite, body weight, and height. There may also be an increase in testicular size. An early morning testosterone sample is obtained 3 weeks after injection to measure endogenous levels. If no physical changes are noted, the dose can be increased by 25–50 mg IM every 4 weeks for another 3 months. Once the testicular volume reaches approximately 10 mL, exogenous testosterone can be discontinued and the HPG axis takes over and allows puberty to continue. If there is no increase in testicular size or no response after a 1 to 2 years of treatment, permanent hypogonadism is diagnosed.