The Neoplastic Appendix
Appendiceal tumors constitute <0.4% of all intestinal neoplasms. They pathologically resemble their small and large intestinal counterparts. The most significant difference between appendiceal and intestinal neoplasms is in the frequency of specific tumor types arising in these sites, with the appendix giving rise to a higher incidence of carcinoid tumors than carcinomas (1). Small appendiceal tumors may obstruct the appendiceal lumen, often causing appendicitis. Nevertheless, because many tumors remain asymptomatic, a diagnosis of neoplasia is seldom made prior to pathologic examination of the resected specimen. The World Health Organization (WHO) classification of appendiceal tumors is shown in Table 9.1 (2). Table 9.2 lists mucinous lesions that may be encountered with appendiceal lesions.
Adenomas
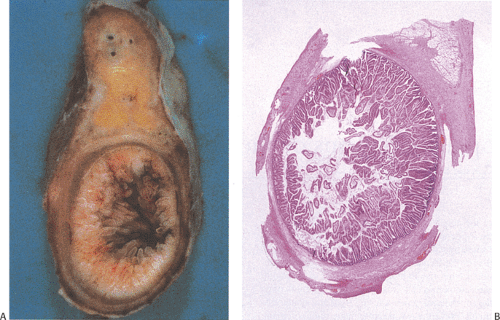
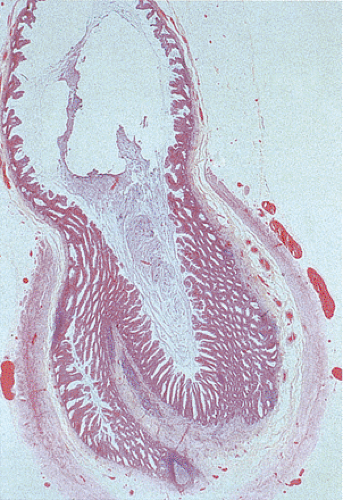
Several types of adenomas develop in the appendix. By far, the most common involve the appendiceal mucosa in a circumferential fashion creating a mucinous cystadenoma (Fig. 9.1); less commonly they grow as a localized lesion resembling their more common colonic counterparts (Fig. 9.2). Both types of adenomas may exhibit tubular, tubulovillous, or villous architectures and the degree of dysplasia can be low grade or high grade. Invasive carcinomas can develop in both. Appendiceal adenomas commonly associate with synchronous colorectal neoplasia (3,4). The molecular features of appendiceal neoplasia are similar to those found in colorectal neoplasms (5).
Mucinous Cystadenomas
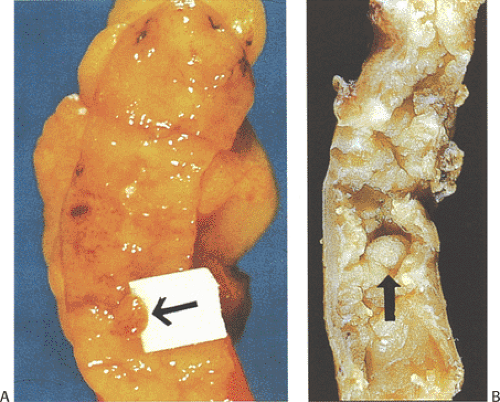
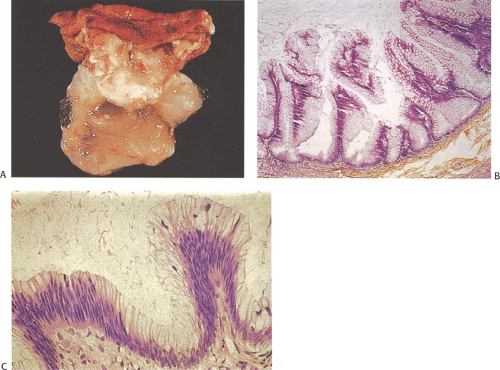
Mucinous cystadenomas (also sometimes referred to as low-grade appendiceal mucinous neoplasms) (6) arise in both males and females, with many series reporting a higher incidence in females. Patients range in age from 27 to 77 years, with an average age of 53 years and a median age of 64 years (7). Approximately 20% of patients have a metachronous or synchronous colonic adenocarcinoma (8). The tumors are usually sporadic lesions but they may complicate longstanding ulcerative colitis. Some patients develop acute appendicitis because the mucin accumulation obstructs the appendiceal lumen (Fig. 9.3). Patients also develop abdominal pain, nausea and vomiting, and, sometimes, a palpable right lower quadrant mass, perforation, or intussusception (Fig. 9.4). Other patients present with pseudomyxoma peritonei or with what appears to be an ovarian tumor.
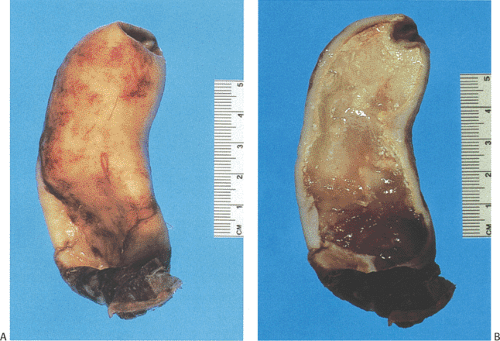
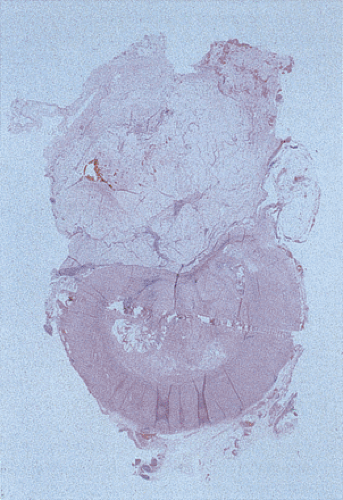
Mucinous cystadenomas can produce large amounts of mucus converting the appendix into a sausage-shaped, cystic or spherical, mucus-filled mass (Fig. 9.3); the average diameter is 2.2 cm with a range of 0.3 to 9 cm (6). Diverticula are often present and there may be areas of rupture. There may also be grossly visible mucus on the serosal surface of the appendix. Mural calcification may produce the gross pattern of a “porcelain appendix” or the obstruction can produce myxoglobulosis, a lesion discussed in Chapter 8.
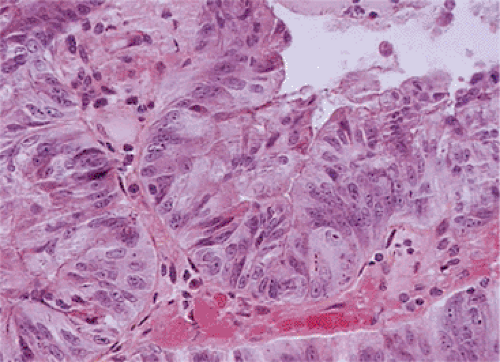
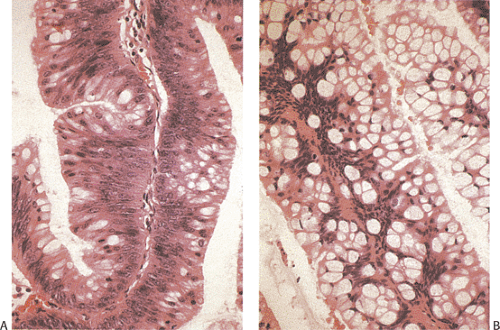
A circumferential proliferation of neoplastic mucinous epithelium replaces the normal epithelium. This neoplastic epithelium may exhibit a full range of neoplasia including low-grade dysplasia, high-grade dysplasia, and invasive carcinoma. Usually a single layer of tall crowded columnar adenomatous epithelium with basally located hyperchromatic, pseudostratified nuclei and clear to eosinophilic cytoplasm lines the neoplastic glands (Fig. 9.5). The elongated nuclei lack nucleoli. Mitotic activity is usually low and usually limited to the base of the glands (7). The glands may be tubular, although a villous architecture may be present. More often the epithelial proliferation produces an undulating pattern. Most mucinous cystadenomas show minimal cytologic atypia, qualifying for a diagnosis of low-grade dysplasia. However, some cases contain moderate to marked atypia and abundant mitotic activity. Areas of high-grade dysplasia (including carcinoma in situ) appear as a disorderly proliferation of cells that lose their polarity (Fig. 9.6) and may exhibit a back-to-back glandular pattern that obliterates the intervening lamina propria. As in the colon, these changes remain confined to the area above the muscularis mucosae. Since the likelihood of an invasive carcinoma increases with the degree of dysplasia, these areas should be well sampled to rule out an invasive process.
The nonneoplastic mucosa often appears atrophic and the usually prominent Peyer patches are often absent. Mucosal denudation is common (Fig. 9.7), either from compression by the intraluminal mucin or by ulceration due to a
coexisting appendicitis. The mucosal ulcers may produce a granulomatous reaction with subsequent mural fibrosis. The intraluminal mucin may compress the lining epithelium in such a way that the flattened epithelium may not be easily recognizable as being neoplastic. A feature that should increase one’s suspicion that an underlying neoplasm is present is the diameter of the appendix. Benign mucoceles seldom measure more than a centimeter in diameter; larger lesions almost always result from an underlying neoplasm. Therefore, these larger mucoceles should be well sampled to rule out the presence of a tumor, if one was not identified in the original sections.
coexisting appendicitis. The mucosal ulcers may produce a granulomatous reaction with subsequent mural fibrosis. The intraluminal mucin may compress the lining epithelium in such a way that the flattened epithelium may not be easily recognizable as being neoplastic. A feature that should increase one’s suspicion that an underlying neoplasm is present is the diameter of the appendix. Benign mucoceles seldom measure more than a centimeter in diameter; larger lesions almost always result from an underlying neoplasm. Therefore, these larger mucoceles should be well sampled to rule out the presence of a tumor, if one was not identified in the original sections.
TABLE 9.1 World Health Organization Classification of Epithelial Nonendocrine Appendiceal Tumors | |
|---|---|
|
TABLE 9.2 Mucinous Lesions of the Appendix and Peritoneum | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
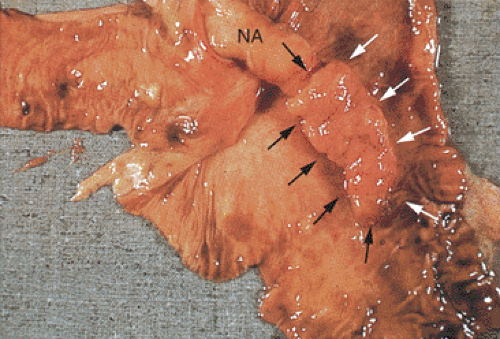
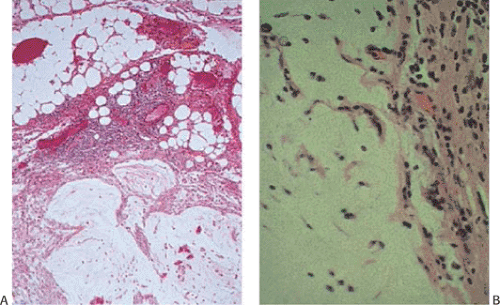
Epithelial or mucinous displacement is common in mucinous cystadenomas due to the pressure from the intraluminal mucin or from a coexisting appendicitis. Increased intraluminal pressure from the mucin can also cause diverticula, ruptures, or fistulae. The wall of the appendix may be fibrotic and significantly attenuated at the rupture site. Mucin with or without epithelial cells may be present in the perforation site and extend to the serosal surface (Fig. 9.8). The serosa itself may show an acute or chronic serositis with inflammation and mesothelial hyperplasia. It is important to search these areas carefully for the presence of both mucin and epithelial cells and to report their presence if they are seen. The histologic features of mucinous cystadenomas with and without extra-appendiceal spread are identical (with the exception of the identification of a breach in the appendiceal wall in the former) (6). The mucus extravasation can be limited to the periappendiceal area or it can spread over large areas of the peritoneal surface (see below). Noninvasive lesions that exhibit marked cytologic atypia or complex intraepithelial proliferations tend to have a higher proliferative rate than low-grade tumors, and if these lesions gain access to the peritoneal cavity, they tend to behave more aggressively than low-grade lesions. We have found that the proliferative rate of the primary tumor as well as its extra-appendiceal extensions is an important prognostic factor.
Neoplastic epithelium can also extend into diverticula (Fig. 9.9), but this does not constitute an invasive carcinoma as evidenced by the presence of lamina propria surrounding the
neoplastic glands. It does not carry the same prognostic significance as serosal mucin extension by dissection or rupture, provided the diverticulum has not ruptured.
neoplastic glands. It does not carry the same prognostic significance as serosal mucin extension by dissection or rupture, provided the diverticulum has not ruptured.
Mucinous cystadenomas are often not appreciated when the appendix is grossed in, especially if there is a coexisting acute appendicitis. Therefore, if a tumor is found, the pathologist should carefully re-examine the appendix for areas of perforation, serosal mucin accumulations, and invasive carcinoma, since these modify the patient’s prognosis and treatment if identified. Perforation sites and serosal mucin deposits should be sampled; the entire tumor should be submitted to rule out invasion. The proximal margin of resection should be identified and submitted for histologic examination. It has also become our practice to obtain a Ki-67 immunostain on the tumor and to report the proliferative rate in the most mitotically active part of the tumor.
The pathology report should indicate the degree of dysplasia in the cystadenoma and whether there is invasive cancer or perforation, whether tumor or mucin is seen outside of the appendix, and the status of the proximal margin. These factors are important because even benign neoplasms that escape the appendix may give rise to subsequent pseudomyxoma peritonei. The prognosis of patients with peritoneal spread is guarded, with just over half of the patients dying after 10 years. Because of the differences in the prognosis, some have proposed the term mucinous borderline tumor of the appendix to reflect the uncertain biology of the lesions (9). These lesions may also be diagnosed as mucinous cystadenoma with mucinosis or cystadenoma with low-grade peritoneal mucinous tumor depending on whether epithelial
cells are identified in the mucin deposits (see below). We, unlike others (3,10), do not believe that cystadenomas with extra-appendiceal extension should be called appendiceal adenocarcinomas, since these patients have a better prognosis than patients with peritoneal carcinomatosis (6,11). Some tumors without evidence of extra-appendiceal extension may also go on to develop pseudomyxoma peritonei. In these cases failure to adequately sample the appendix to detect the tumor extension is the likely explanation.
cells are identified in the mucin deposits (see below). We, unlike others (3,10), do not believe that cystadenomas with extra-appendiceal extension should be called appendiceal adenocarcinomas, since these patients have a better prognosis than patients with peritoneal carcinomatosis (6,11). Some tumors without evidence of extra-appendiceal extension may also go on to develop pseudomyxoma peritonei. In these cases failure to adequately sample the appendix to detect the tumor extension is the likely explanation.
Mucinous Cystadenoma Coexisting with Carcinoid Tumor
Occasionally one sees an appendiceal mucinous cystadenoma coexisting with an appendiceal carcinoid tumor. The carcinoid and the epithelial components may lie side by side or may arise in different parts of the appendix. Carr et al used the term dual carcinoid epithelial neoplasia to describe this phenomenon (12). One usually does not see intermediate histologies between the epithelial and neuroendocrine tumors. The carcinoid tumor may have the histologic features of any appendiceal carcinoid (see Chapter 17). Therefore, they may be trabecular, tubular, or goblet cell in type. The prognosis is determined by the histologic features of each of the components.
Localized Adenomas
Isolated sessile or pedunculated adenomas resembling their colonic counterparts occur, but it is difficult to assess their incidence because these lesions tend to remain asymptomatic and most pathologists do not open the appendix along its long axis.
The adenomas affect patients of all ages, including children (13); median patient age is the mid-50s (14). Adenomas occur in younger individuals in patients with familial polyposis. It is important to remember that patients with appendiceal adenomas often have additional primary tumors at other sites including the colon, breast, kidney, ovary, and gallbladder (14,15,16).
The adenomas affect patients of all ages, including children (13); median patient age is the mid-50s (14). Adenomas occur in younger individuals in patients with familial polyposis. It is important to remember that patients with appendiceal adenomas often have additional primary tumors at other sites including the colon, breast, kidney, ovary, and gallbladder (14,15,16).
Isolated adenomas are generally discovered incidentally in appendices removed for other reasons. They may also be detected at the time of colonoscopy if they prolapse through the appendiceal orifice into the cecum. Larger sessile adenomas produce appendicitis by obstructing the appendiceal lumen. They may also produce diverticula (Fig. 8.6). These adenomas histologically resemble colorectal adenomas (see Chapter 14); the evolution of an adenoma into a carcinoma follows the same sequence. Sporadic adenomas may be tubular, tubulovillous, or villous in architecture and contain varying degrees of dysplasia (Fig. 9.10). Grossly invisible tubular adenomas occur in patients with familial polyposis. As in the colon, a tumor must invade through the muscularis mucosae into the underlying submucosa in order to diagnose an invasive cancer. Simple appendectomy represents adequate treatment for patients with adenomas that do not contain an invasive cancer.
Mixed Hyperplastic–Adenomatous Polyps, Serrated Adenomas, and Sessile Serrated Polyps
Mixed hyperplastic–adenomatous polyps, serrated adenomas, and sessile serrated polyps can develop in the appendix. As with traditional adenomas, the histologic features of these lesions resemble their colonic counterparts, and the features of the mixed lesions are discussed in Chapter 14.
Serrated adenomas represent a distinctive form of adenomatous polyp with a tendency to develop in the appendix and right colon (17). The polyp architecture reminds one of a hyperplastic polyp because of the presence of serrated lumens (Fig. 9.11), but the cytologic features differ from those seen in hyperplastic polyps in that the often eosinophilic cells appear immature and the hyperdistended goblet cells and thickened collagen table typically present in hyperplastic polyps are absent. The epithelium usually contains more mucin than seen in typical adenomas but less than one sees in mucinous cystadenomas. The nuclei appear pseudostratified and elongated in comparison to the basally located, rounder nuclei typical of hyperplastic polyps. Mitotic activity is increased over that seen in hyperplastic polyps and mitoses can be identified at the free surface in serrated adenomas (17). The lesion may exhibit a high level of microsatellite instability (18).
Sessile serrated polyps also develop in the appendix, although they are seldom reported as such, especially in the older literature. The lesions described by Younes et al are an example of this lesion (19). Sessile serrated polyps superficially resemble hyperplastic polyps but they tend to be larger than the usual hyperplastic polyp, sometimes covering extensive areas of the mucosa. Typically, the glands appear serrated and the serrations extend deeper into the crypt than the usual hyperplastic polyp. The base of the glands tends to
extend sideways above the muscularis mucosae and the proliferative zone is asymmetric in appearance. The cells lining the glands do not appear to be obviously adenomatous, but they are crowded and contain variable amounts of mucin. These appendiceal lesions have a high association with right-sided colonic carcinomas (19). These three lesions are discussed in greater detail in Chapter 14.
extend sideways above the muscularis mucosae and the proliferative zone is asymmetric in appearance. The cells lining the glands do not appear to be obviously adenomatous, but they are crowded and contain variable amounts of mucin. These appendiceal lesions have a high association with right-sided colonic carcinomas (19). These three lesions are discussed in greater detail in Chapter 14.
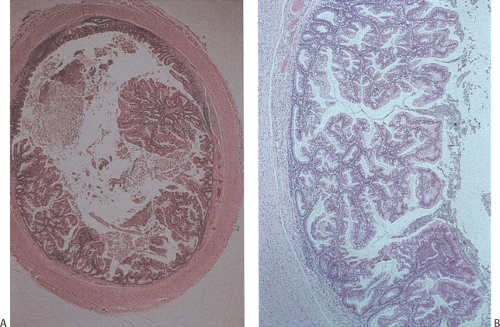 FIG. 9.11. Serrated adenoma A: Low-power magnification demonstrating the presence of an almost completely circumferential serrated adenoma. B: Higher magnification demonstrating the presence of glands with a serrated architecture. Closer examination of the lesion shows the presence of serrated glandular lumens lined by cells that have histologic features between those of a classic adenoma or hyperplastic polyp.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|