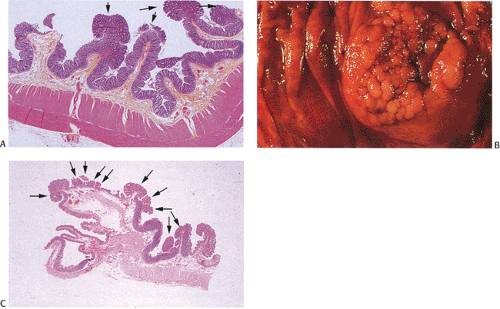
Adenomas
Adenomas, the benign glandular neoplasms that precede colon cancer development, originate from the intestinal epithelium. They occur singly or multiply. When multiple, the patients may have a genetic syndrome (see Chapter 12).
Biologic Alterations in Adenomas
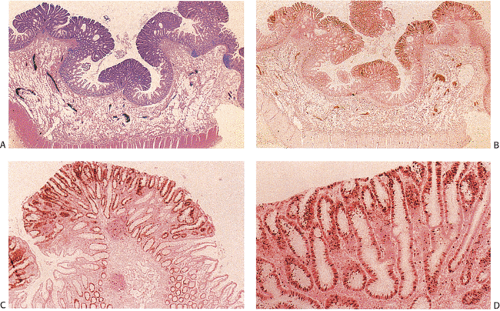
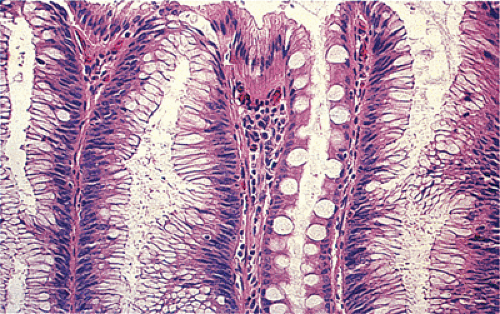
Despite their differing structure, all adenomas share two basic features of neoplasia, dysregulated proliferation and the failure to fully differentiate. The dysregulated proliferation is evidenced by an upward shift in the proliferative compartment (Fig. 14.5). This shift can be highlighted with the use of proliferation markers, such as with the antibody MIB-1 (Fig. 14.6) or by other labeling techniques. Mitotic figures, including abnormal ones, are present throughout the entire length of the hyperchromatic, adenomatous epithelium.
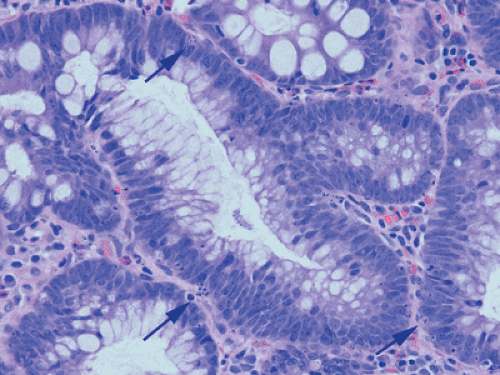
It has always been believed that adenomas form because the rate of cellular proliferation exceeds that of cellular exfoliation. However, today we know that cellular exfoliation is not the only mechanism for removing cells from the crypt. Cells also undergo apoptosis. In the normal colon, most apoptosis occurs near the luminal surface (159) consistent with a model of colorectal cell differentiation and senescence, which culminates in physiologic cell death as the cells migrate upward from the proliferative compartment in the basal crypt. This process is under the influence of the autocrine growth inhibitory apoptosis-inducing effect of transforming growth factor-β (TGF-β) (160). In contrast to the normal crypt, adenomas contain numerous apoptotic
cells (Fig. 14.7), which often lie at the adenoma base, a reversal of the normal distribution. This observation led Moss et al (161) to suggest that adenomas exhibit a reversed epithelial cell migration and an inward growth pattern directed toward the crypt base rather than toward the lumen. The shift in the location of the apoptotic figures is accompanied by a shift in TGF-β–immunoreactive cells from the adenoma surface to the base. The apoptosis-related bcl-2 family of proteins also exhibit altered expression in adenomas (162,163,164).
cells (Fig. 14.7), which often lie at the adenoma base, a reversal of the normal distribution. This observation led Moss et al (161) to suggest that adenomas exhibit a reversed epithelial cell migration and an inward growth pattern directed toward the crypt base rather than toward the lumen. The shift in the location of the apoptotic figures is accompanied by a shift in TGF-β–immunoreactive cells from the adenoma surface to the base. The apoptosis-related bcl-2 family of proteins also exhibit altered expression in adenomas (162,163,164).
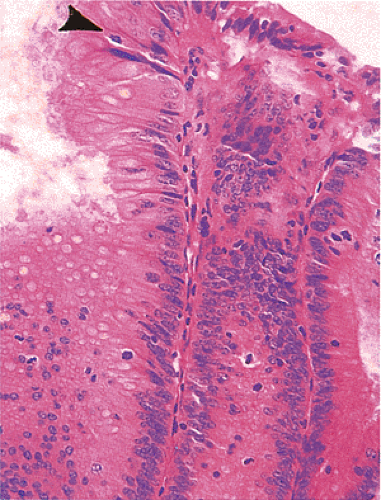 FIG. 14.5. The surface of an adenoma demonstrating the presence of increased numbers of mitoses. One mitotic figure is near the top of the mucosa (arrowhead). |
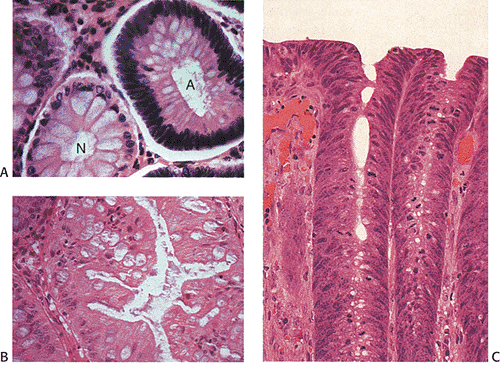
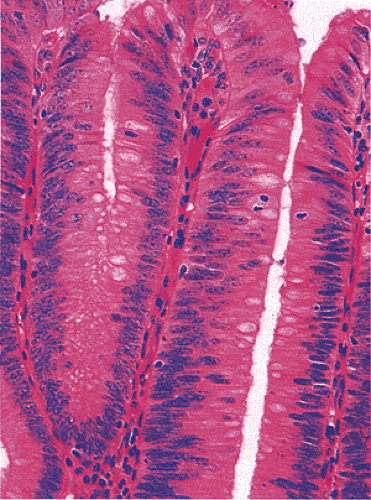
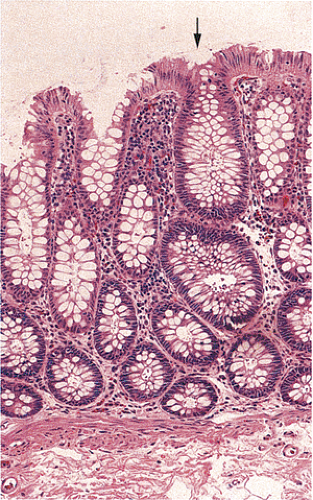
Adenomas also show abnormalities in epithelial cell differentiation. Morphologically and phenotypically, adenomatous epithelium resembles replicating cells normally present in the crypt base. Tall cells with prominent, elongated, hyperchromatic nuclei produce a characteristic “picket fence” pattern (Figs. 14.7, 14.8, 14.9) as they line the adenomatous glands. The adenomatous epithelium contains incompletely differentiated goblet cells and absorptive cells at all levels of the crypt, including the free surface (Fig. 14.9). This adenomatous pattern contrasts with the pattern of progressive differentiation seen in normal crypts. Normally, cells at the crypt base appear basophilic. As they move up the crypt toward the lumen, the cells become larger and increasingly more
eosinophilic. Mitoses disappear. A mixed cell population is present (Fig. 14.10). A gradient of differentiation appears, which is most evident histologically in the goblet cells, since goblet cells acquire increasingly larger supranuclear mucin accumulations as they mature.
eosinophilic. Mitoses disappear. A mixed cell population is present (Fig. 14.10). A gradient of differentiation appears, which is most evident histologically in the goblet cells, since goblet cells acquire increasingly larger supranuclear mucin accumulations as they mature.
Adenoma Growth
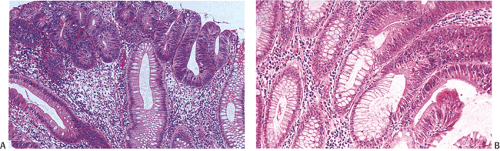
Small adenomas represent neoplastic clonal populations of colonic epithelial cells, suggesting that they arise from a single abnormal precursor stem cell. Adenomas begin in a single crypt (Fig. 14.11) and then grow by replacing normal epithelium in a centrifugal manner. Unicryptal adenomas are rare and most typically affect patients with FAP (see Chapter 12). New adenomatous glands result from infolding of the neoplastic surface epithelium. The neoplastic cells appear to cluster at the luminal aspect of the mucosa without extending to the base of the glands (Fig. 14.12). Normal-appearing mucosa lies below the adenomatous glands (Fig. 14.12). In 86% of early tubular adenomas, the number of gland openings along the polyp surface is larger than the number of gland bases; this difference increases with polyp size (165). Additionally, gland proliferation predominates in the upper crypts and along the surface of the lesions (Fig. 14.6). Early adenomas present as small growths with a very benign tubular histology.
A small proportion of tubular adenomas increases in size and develop villous features and cytologic characteristics of high-grade dysplasia. The progression of most small adenomas is slow and occurs over several years. On average, small adenomas double their diameter in 10 years (166). In one study, small adenomas doubled during a 2-year observation period, but none of the adenomas observed over time exceeded 5 mm in size at the time of resection, nor did any show high-grade dysplasia or carcinoma (166). Another estimate of the projected increased growth relates to lesional volume. There is a 52% mean volume increase after 2 years (166).
Some adenomas ultimately progress to invasive cancers. However, it should be remembered that not all adenomas progress, and that some stay stable or may even regress and disappear while new ones form in the same colon (167).
Incidence
Incidence rates of adenomas vary considerably throughout the world. Geographic areas exhibiting a high risk for colon cancer also exhibit a high risk for adenoma development and
vice versa. The incidence in the general population varies from 0% to 69% depending on the country of origin (168,169,170) and on how the adenomas are detected (171). The incidence of adenomas depends on several other factors, including (a) whether one is examining data from an autopsy study versus an endoscopic screening study, (b) the age of the patient, and (c) whether or not the patient has a hereditary colon cancer syndrome.
vice versa. The incidence in the general population varies from 0% to 69% depending on the country of origin (168,169,170) and on how the adenomas are detected (171). The incidence of adenomas depends on several other factors, including (a) whether one is examining data from an autopsy study versus an endoscopic screening study, (b) the age of the patient, and (c) whether or not the patient has a hereditary colon cancer syndrome.
Some patient populations have an extremely low adenoma incidence. For example, no adenomas were found among 14,000 autopsies performed on the South African Bantu (172), and Medillin, Colombia, which has a low incidence of colorectal cancer, also has a low incidence of adenomas (173). In Western populations, the average prevalence rate for adenomas from flexible sigmoidoscopy screening is 10%, and colonoscopic screening prevalence averages 25% (174). Adenomas accounted for 68% of all polyps removed by colonoscopy in the National Polyp Study (175). In the 50- to 59-year age group, population screening studies and autopsy studies show an adenoma prevalence rate of 41.3% to 69% (176), increasing in advancing years up to 88% in centenarians (177). Arminsky and McLean (178) documented a 7.5% increase in adenoma incidence per decade.
These data contrast with incidences of only 2.8% to 50% and a mean of 10% among autopsied patients (179,180). Low prevalence rates from autopsy series reflect geographic variations and various methodologic biases, including the fact that usually the autopsies are performed by numerous individuals with colorectal examinations ranging from a casual inspection to examinations with a hand lens. The
most reliable autopsy data derive from those studies performed by a single, experienced investigator. Another confounding variable is whether the data include autopsies on children as well as on adults. Autopsy series containing large numbers of children would be expected to have lower prevalence rates due to the fact that adenomas are age-related lesions. In carefully performed studies, 46.9% to 69% of cases have at least one adenoma (178,181).
most reliable autopsy data derive from those studies performed by a single, experienced investigator. Another confounding variable is whether the data include autopsies on children as well as on adults. Autopsy series containing large numbers of children would be expected to have lower prevalence rates due to the fact that adenomas are age-related lesions. In carefully performed studies, 46.9% to 69% of cases have at least one adenoma (178,181).
Patient Age and Sex
Age and male sex correlate with adenoma development (170,175,182). Adenomas show a sharp rise in incidence in patients without hereditary adenomatous polyp syndromes at about age 40, and adenoma incidence peaks at age 60 or 70 years. In the National Polyp Study, adenomas occurred more frequently in men than women, 61.6% versus 38.4%. Patient mean age was 62 6 11 years. Of these, 21% reported a family history of colon cancer and 12% had a family history of colonic polyps. Forty-three percent had a family history of another cancer. Thirty-nine percent had more than one adenoma (175). These authors found that the prevalence of adenomas increased 21% in the 6th to the 8th decades (53%). The prevalence of polyps in patients aged 50 to 70 years is 30% to 50%, and the likelihood of cancer developing is 6% (183).
Lesion Location
Based on endoscopic studies, most sporadic adenomas arise in the rectosigmoid (66% to 77%), where 97% are amenable to endoscopic removal (184). However, following endoscopic removal of all adenomas from the colon, adenomas have a higher incidence on the right side of the bowel in the initial follow-up period and then become more evenly distributed with time (184). Adenomas also shift from a distal to a proximal location as patients age (168,169,170,171,185). Thus, left-sided adenomas occur more commonly in younger age groups and right-sided lesions increase in frequency in individuals older than 65 years of age. HNPCC patients have a predominance of right-sided adenomas at all ages. FAP patients have predominantly left-sided lesions and, since patients undergo prophylactic colectomy, it is unknown whether the adenoma distribution would shift toward right-sided lesions with age.
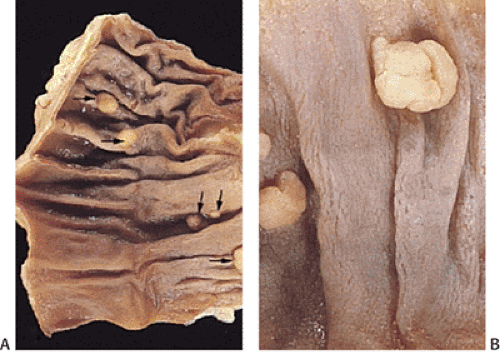
Some adenomas cluster (Fig. 14.13). This means that multiple adenomas tend to occur closer together than would be expected from the general distribution of adenomas. This phenomenon occurs in all colonic segments but is less pronounced in the rectum than in other parts of the large intestine (186).
Frequency of Multiple Polyps
Individuals with one adenoma have a 40% to 55% likelihood of having additional synchronous lesions (184,187,188). The additional adenomas are detected at the same time as the initial adenoma (synchronous adenomas) or at a different time (metachronous adenomas). The prevalence of multiple adenomas increases with age. Nine percent of individuals younger than 60 years, 17% of people between 60 and 74 years, and 28% of people older than 75 years have three or more adenomas. Multiple adenomas also arise with increased frequency at ureterosigmoidostomy sites and in patients with hereditary colon cancer syndromes and FAP. Sometimes a single sessile adenoma may appear as multiple adenomas. It may be difficult to separate a single lesion from multiple lesions if one is unaware of the gross appearance of the lesion (Fig. 14.14).
The incidence of synchronous neoplasms in patients with an index rectosigmoid adenoma measuring <5 mm in diameter, with an index rectosigmoid adenoma measuring >5 mm, or with a rectosigmoid carcinoma is 34%, 53%, and 73%, respectively (188). The synchronous neoplasm is an adenoma measuring >5 mm in diameter is 13%, 40%, and 64%, respectively (188). The incidence of large intestinal adenomas occurring synchronously with carcinomas is approximately double that of adenomas occurring alone. Metachronous adenomas, compared to index adenomas,
tend to be smaller, tubular, only mildly dysplastic, and more uniformly distributed (189). The substantial prevalence of proximal colonic neoplasms, including advanced lesions, in asymptomatic, average-risk patients with rectosigmoid adenomas measuring <5 mm in diameter warrants colonoscopy in these patients in order to detect them (189).
tend to be smaller, tubular, only mildly dysplastic, and more uniformly distributed (189). The substantial prevalence of proximal colonic neoplasms, including advanced lesions, in asymptomatic, average-risk patients with rectosigmoid adenomas measuring <5 mm in diameter warrants colonoscopy in these patients in order to detect them (189).
A relationship exists between adenoma multiplicity and histologic findings. In patients with a single adenoma, 38.8% are villous, whereas those with multiple adenomas have a 60.1% chance of having at least one villous adenoma (190). Patients with multiple adenomas are also more likely to harbor at least one adenoma that contains high-grade dysplasia (13.8%) versus patients with a single adenoma (7.3%).
Recurrent Adenomas
“Recurrent” adenomas result from the appearance of new adenomas, continued growth of an incompletely resected adenoma, or detection of a previously undetected but pre-existing adenoma. The overall recurrence rates for new adenomas are
estimated at 20% to 60% with average follow-up times of 3 to 10 years after index polypectomy (171,183,184,191). Most recurrences occur in the first 2 years following polypectomy. The estimated time to finding new adenomas is 58 months for patients clear on the first colonoscopy and 16 months for patients who had adenomas on the first examination. In 18% of patients, the adenomas arise proximal to the splenic flexure (192,193). Villous tumors, particularly broadly sessile ones, usually have a less well-defined border than do tubular adenomas and, therefore, have a greater tendency to recur after local resection than smaller, pedunculated adenomas.
estimated at 20% to 60% with average follow-up times of 3 to 10 years after index polypectomy (171,183,184,191). Most recurrences occur in the first 2 years following polypectomy. The estimated time to finding new adenomas is 58 months for patients clear on the first colonoscopy and 16 months for patients who had adenomas on the first examination. In 18% of patients, the adenomas arise proximal to the splenic flexure (192,193). Villous tumors, particularly broadly sessile ones, usually have a less well-defined border than do tubular adenomas and, therefore, have a greater tendency to recur after local resection than smaller, pedunculated adenomas.
Endoscopic follow-up studies to evaluate new adenomas are hampered by the fact that as many as 25% to 27% of adenomas measuring <5 mm in diameter and up to 6% of adenomas measuring 1 cm in diameter are missed during one endoscopic examination (194,195). Right-sided adenomas are missed more often (27%) than left-sided adenomas (21%) (194). Lesions measuring up to 8 cm in diameter may be missed, and some of these may contain areas of malignancy (196).
Adenoma Incidence in Hereditary Colon Cancer Syndromes
Relatives of individuals with colorectal cancer have an adenoma prevalence rate of 39%. Patients with FAP or its variants (see Chapter 12) have an increased incidence of colonic adenomas. These are multiple and occur at a younger age than they do in the general population. FAP patients have the largest number of adenomas, although some patients have the attenuated form of the disease. Thirty percent of HNPCC patients have at least one adenoma and 20% have multiple adenomas. It is unusual to find more than five adenomas in HNPCC patients (197,198). Table 14.7 compares polyp numbers in the HNPCC and FAP hereditary syndromes.
Clinical Features and Diagnosis
Adenomas develop in diverse clinical settings. They either occur sporadically or arise in the setting of a hereditary syndrome such as a polyposis syndrome (FAP) or HNPCC. Sometimes the clinical features result from associated diseases such as diverticulosis (diverticulitis). Small adenomas, ranging up to 1.0 cm in maximum diameter, usually remain asymptomatic unless they are located in the rectosigmoid, in which case they may bleed when their surfaces become traumatized by the passage of well-formed, hardened stool. Larger lesions become symptomatic, with the symptoms depending on polyp size and location. Bleeding is the most frequent symptom, followed by a profuse, watery, or mucoid rectal discharge. Bleeding occurs more often in left-sided lesions than right-sided ones (199). At most, the patient may notice a slight reddish discoloration of the stool after defecating. The bleeding is seldom severe. Factors correlating with severe hemorrhage include adenoma size, pedunculation, and a villous growth pattern (199). The incidence of bleeding increases with increasing adenoma size and once a carcinoma develops within the adenoma. Villous tumors are more likely to bleed than tubular ones, since they tend to be larger and have a statistically higher incidence of coexisting carcinoma. The most common symptoms of villous lesions include bleeding, mucous diarrhea, constipation, and tenesmus (200). Some adenomas lead to incontinence, prolapse, and anemia. If the adenomas are large enough, they may cause changes in bowel habits or intussusception. Cecal lesions that block the appendiceal orifice may produce symptoms mimicking acute appendicitis. Ominous signs and symptoms associated with adenomas include obstruction and abdominal pain.
TABLE 14.7 Comparison of Hereditary Nonpolyposis Colon Cancer (HNPCC) and Familial Adenomatous Polyposis (FAP) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Other clinical features develop in individuals with distinctive polyposis syndromes in whom extraintestinal manifestations may herald the presence of intestinal lesions. These are discussed in Chapter 12.
Endoscopic Features
Small (<5 mm) colorectal polyps commonly affect individuals older than 50 years of age (201) and adenomas account for 60% to 66% of these small lesions (167,202,203,204). The
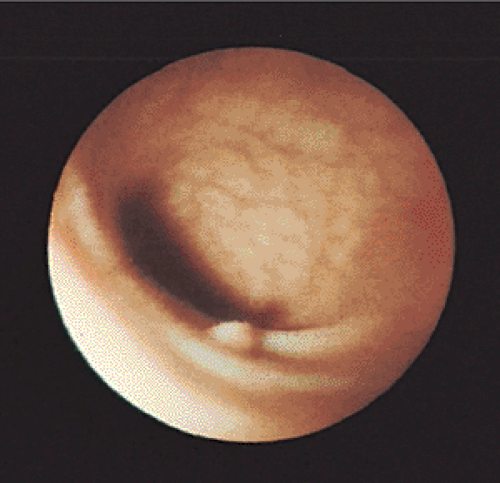
gross endoscopic appearance may suggest the correct diagnosis. However, the endoscopic diagnosis is only correct in 82% of smaller polyps (Fig. 14.15), so histologic examination is required for confirmation (205). The current wisdom is that distal small adenomas represent biomarkers of risk for colorectal neoplasia, warranting a complete colonoscopy in the patient and lifetime surveillance for colon cancer (167). Recently, attention has focused on the development of fluorescence endoscopic imaging and high-resolution chromoendoscopy, which might provide morphologic detail of diminutive colorectal polyps that might correlate with polyp histology and eliminate the need for a biopsy and/or subsequent colonoscopy.
gross endoscopic appearance may suggest the correct diagnosis. However, the endoscopic diagnosis is only correct in 82% of smaller polyps (Fig. 14.15), so histologic examination is required for confirmation (205). The current wisdom is that distal small adenomas represent biomarkers of risk for colorectal neoplasia, warranting a complete colonoscopy in the patient and lifetime surveillance for colon cancer (167). Recently, attention has focused on the development of fluorescence endoscopic imaging and high-resolution chromoendoscopy, which might provide morphologic detail of diminutive colorectal polyps that might correlate with polyp histology and eliminate the need for a biopsy and/or subsequent colonoscopy.
Gross Features
Grossly, adenomas assume one of three major growth patterns: (a) pedunculated, (b) sessile, or (c) flat or depressed (Fig. 14.16).
Pedunculated and Sessile Adenomas
Most sporadic colorectal adenomas appear as exophytic, mucosal protrusions. They range in size from invisible
unicryptal lesions to large sessile adenomas sometimes measuring >20 cm in greatest dimension. Adenoma size generally correlates with gross growth pattern.
unicryptal lesions to large sessile adenomas sometimes measuring >20 cm in greatest dimension. Adenoma size generally correlates with gross growth pattern.
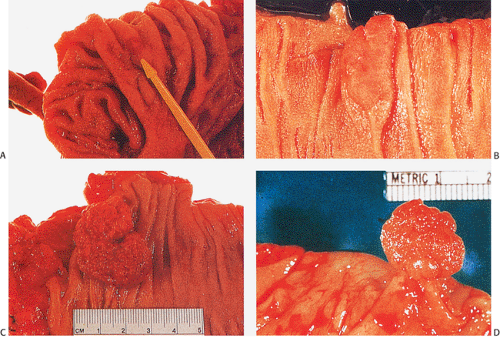 FIG. 14.16. Gross appearance of adenomatous polyps. A: Small adenoma on mucosal fold. B: Larger plaquelike lesion. C: Large sessile raspberrylike lesion. D: Pedunculated adenoma. |
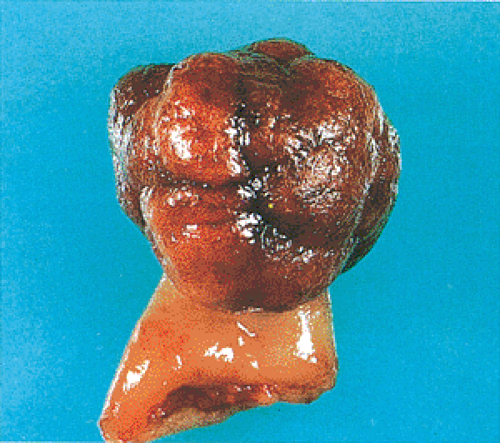 FIG. 14.17. Gross appearance of a pedunculated adenoma with the typical lobulated head and a stalk covered by normal mucosa. |
Adenomas measuring only 1 or 2 mm grossly resemble hyperplastic polyps. These minute adenomas have smooth surfaces and lack lobulations, and their color often resembles that of the normal mucosa. However, when such lesions are examined under a dissecting microscope, they exhibit characteristic pit patterns that differ from those seen in either small carcinomas or hyperplastic polyps.
Polyp architecture depends in part on whether the adenoma has a tubular, villous, or tubulovillous histologic pattern. The typical tubular adenoma presents as a small, spherical, and variably pedunculated lesion, with its surface broken into lobules by intercommunicating clefts in larger lesions (Figs. 14.16 and 14.17). Larger lesions appear redder than the surrounding mucosa (Fig. 14.17), unless the patient has melanosis coli, in which case the lesions may appear lighter. Larger lesions exhibit a lobulated, bosselated or villous, raspberrylike, friable surface. In surgical material, approximately 90% of adenomas appear variably pedunculated and normal mucosa lines their stalks. The stalk ranges in length from several millimeters to a few centimeters. Tubulovillous adenomas tend to be larger than tubular adenomas, with a mean diameter of 19.0 mm (178).
 FIG. 14.18. Villous adenomas are composed of many fingerlike fronds that give them a shaggy appearance. |
Villous adenomas fall into three types: (a) flat, carpetlike masses; (b) lobulated, bulky, sessile masses; and (c) pedunculated lesions with short, broad pedicles. Sessile adenomas tend to be large, shaggy lesions covered by fingerlike fronds (Figs. 14.18 and 14.19). Generally, adenomas appear as grossly homogeneous, soft lesions without induration, ulceration, or fixation. Areas of pigmentation
may indicate previous hemorrhage, fibrosis, pseudoinvasion, or previous fulguration (Fig. 14.20). Areas of ulceration, depression, or firmness suggest the possibility of a coexisting carcinoma (Fig. 14.21). Villous adenomas can be multiple and often associate with other adenomas, polyps, or separate carcinomas.
may indicate previous hemorrhage, fibrosis, pseudoinvasion, or previous fulguration (Fig. 14.20). Areas of ulceration, depression, or firmness suggest the possibility of a coexisting carcinoma (Fig. 14.21). Villous adenomas can be multiple and often associate with other adenomas, polyps, or separate carcinomas.
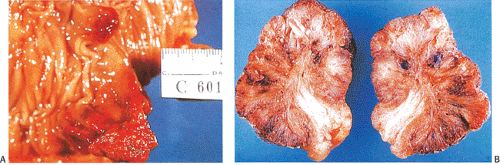 FIG. 14.20. Hemorrhage within polyps. A: The entire surface of these two polyps is markedly reddened due to hemorrhage. B: Cut surface of a polyp with hemorrhage and secondary fibrosis.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|